Sofosbuvir and Ribavirin for Treatment of Chronic Hepatitis C in Patients Coinfected With Hepatitis C Virus and HIV: The Impact on Patient-Reported Outcomes
- PMID: 25583164
- PMCID: PMC5007583
- DOI: 10.1093/infdis/jiv005
Sofosbuvir and Ribavirin for Treatment of Chronic Hepatitis C in Patients Coinfected With Hepatitis C Virus and HIV: The Impact on Patient-Reported Outcomes
Abstract
Background: Sofosbuvir-containing regimens have been approved for treatment of hepatitis C virus (HCV) infection in human immunodeficiency virus (HIV)-infected patients. We assessed the effect of treatment with sofosbuvir and ribavirin on patient-reported outcomes (PROs) in individuals with HIV/HCV coinfection.
Methods: HIV/HCV-coinfected patients were treated for 12 or 24 weeks with sofosbuvir and ribavirin. Matched HCV-monoinfected controls were also evaluated. All subjects completed standard PRO questionnaires before, during, and after treatment.
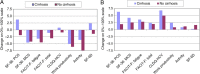
Results: Included were 497 participants from the PHOTON-1 and PHOTON-2 clinical trials. At baseline, more impairment in PRO scores was noted in HIV/HCV-coinfected patients, compared with HCV-monoinfected patients. During treatment, moderate decrements in PRO scores (change, up to -6.8% on a 0%-100% scale; P = .0053) were experienced regardless of treatment duration and were similar to those for HCV-monoinfected patients (all P > .05). In 413 HIV/HCV-coinfected patients with a virologic response sustained for 12 weeks after treatment cessation, most PRO scores improved (change, up to +7.6%; P < .0001), similar to findings for HCV-monoinfected patients. In multivariate analysis, in addition to clinico-demographic predictors, coinfection with HIV was associated with PRO impairment at baseline (beta, up to -7.6%; P < .002) but not with treatment-emergent changes in PRO scores (all P > .05).
Conclusions: Patients with HIV/HCV coinfection tolerate interferon-free sofosbuvir-based anti-HCV regimens well and, despite the presence of some baseline impairment, have treatment-emergent changes in PRO scores that are similar to those of patients with HCV monoinfection.
Clinical trials registration: NCT01667731 (PHOTON-1), NCT01783678 (PHOTON-2), NCT01604850 (FUSION), and NCT01682720 (VALENCE).
Keywords: HCV/HIV coinfection; Hepatitis C treatment; cirrhosis; health-related quality of life; patient-reported outcomes; ribavirin; sofosbuvir.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Hepatitis C Virus Infection Is Systemic: Meeting Additional Goals.J Infect Dis. 2015 Aug 1;212(3):343-4. doi: 10.1093/infdis/jiv006. Epub 2015 Jan 12. J Infect Dis. 2015. PMID: 25583165 No abstract available.
References
-
- Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 2014; 39:518–31. - PubMed
-
- Kallman J, O'Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci 2007; 52:2531–9. - PubMed
-
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

