BRCA1-IRIS inactivation overcomes paclitaxel resistance in triple negative breast cancers
- PMID: 25583261
- PMCID: PMC4322455
- DOI: 10.1186/s13058-014-0512-9
BRCA1-IRIS inactivation overcomes paclitaxel resistance in triple negative breast cancers
Abstract
Introduction: Intrinsic or acquired chemoresistance is a major problem in oncology. Although highly responsive to chemotherapies such as paclitaxel, most triple negative breast cancer (TNBC) patients develop chemoresistance. Here we investigate the role of BRCA1-IRIS as a novel treatment target for TNBCs and their paclitaxel-resistant recurrences.
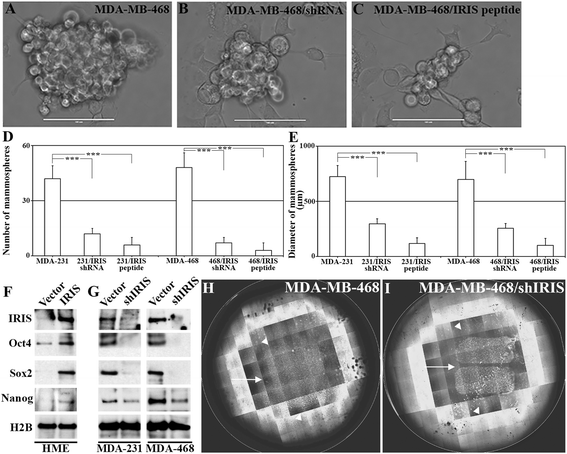
Methods: We analyzed the response of BRCA1-IRIS overexpressing normal mammary cells or established TNBC cells silenced from BRCA1-IRIS to paclitaxel in vitro and in vivo. We analyzed BRCA1-IRIS downstream signaling pathways in relation to paclitaxel treatment. We also analyzed a large cohort of breast tumor samples for BRCA1-IRIS, Forkhead box class O3a (FOXO3a) and survivin expression. Finally, we analyzed the effect of BRCA1-IRIS silencing or inactivation on TNBCs formation, maintenance and response to paclitaxel in an orthotopic model.
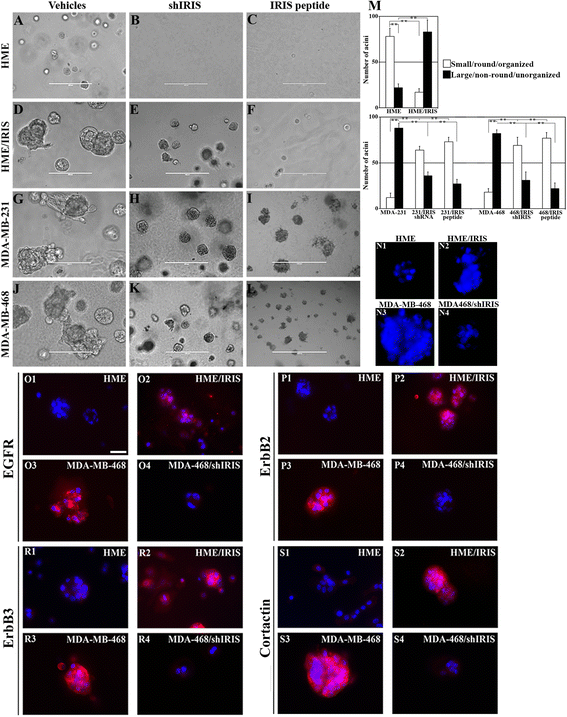
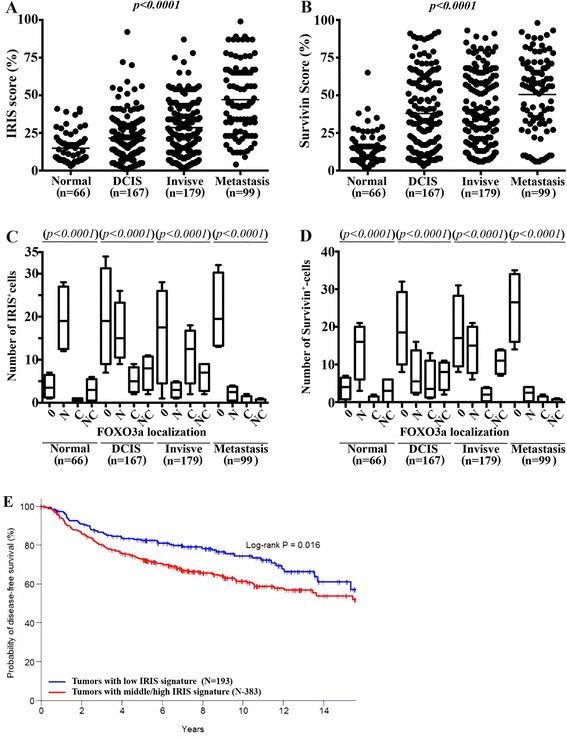
Results: We show that low concentrations of paclitaxel triggers BRCA1-IRIS expression in vitro and in vivo, and that BRCA1-IRIS activates two autocrine signaling loops (epidermal growth factor (EGF)/EGF receptor 1 (EGFR)-EGF receptor 2 (ErbB2) and neurogulin 1 (NRG1)/ErbB2-EGF receptor 3 (ErbB3), which enhances protein kinase B (AKT) and thus survivin expression/activation through promoting FOXO3a degradation. This signaling pathway is intact in TNBCs endogenously overexpressing BRCA1-IRIS. These events trigger the intrinsic and acquired paclitaxel resistance phenotype known for BRCA1-IRIS-overexpressing TNBCs. Inactivating BRCA1-IRIS signaling using a novel inhibitory mimetic peptide inactivates these autocrine loops, AKT and survivin activity/expression, in part by restoring FOXO3a expression, and sensitizes TNBC cells to low paclitaxel concentrations in vitro and in vivo. Finally, we show BRCA1-IRIS and survivin overexpression is correlated with lack of FOXO3a expression in a large cohort of primary tumor samples, and that BRCA1-IRIS overexpression-induced signature is associated with decreased disease free survival in heavily treated estrogen receptor alpha-negative patients.
Conclusions: In addition to driving TNBC tumor formation, BRCA1-IRIS overexpression drives their intrinsic and acquired paclitaxel resistance, partly by activating autocrine signaling loops EGF/EGFR-ErbB2 and NRG1/ErbB2-ErbB3. These loops activate AKT, causing FOXO3a degradation and survivin overexpression. Taken together, this underscores the need for BRCA1-IRIS-specific therapy and strongly suggests that BRCA1-IRIS and/or signaling loops activated by it could be rational therapeutic targets for advanced TNBCs.
Figures




References
-
- Ozols RF. Paclitaxel (Taxol)/carboplatin combination chemotherapy in the treatment of advanced ovarian cancer. Semin Oncol. 2000;27:3–7. - PubMed
-
- Jiménez B, Trigo JM, Pajares BI, Sáez MI, Quero C, Navarro V, et al. Efficacy and safety of weekly paclitaxel combined with cetuximab in the treatment of pretreated recurrent/metastatic head and neck cancer patients. Oral Oncol. 2013;49:182–185. doi: 10.1016/j.oraloncology.2012.09.003. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

