Investigation of the binding and functional properties of extended length D3 dopamine receptor-selective antagonists
- PMID: 25583363
- PMCID: PMC4449328
- DOI: 10.1016/j.euroneuro.2014.11.013
Investigation of the binding and functional properties of extended length D3 dopamine receptor-selective antagonists
Abstract
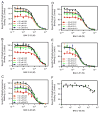
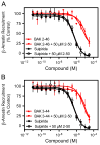
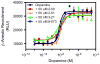
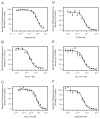
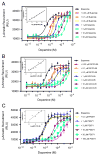
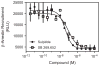
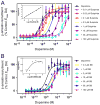
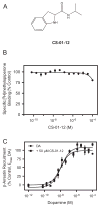
The D3 dopamine receptor represents an important target in drug addiction in that reducing receptor activity may attenuate the self-administration of drugs and/or disrupt drug or cue-induced relapse. Medicinal chemistry efforts have led to the development of D3 preferring antagonists and partial agonists that are >100-fold selective vs. the closely related D2 receptor, as best exemplified by extended-length 4-phenylpiperazine derivatives. Based on the D3 receptor crystal structure, these molecules are known to dock to two sites on the receptor where the 4-phenylpiperazine moiety binds to the orthosteric site and an extended aryl amide moiety docks to a secondary binding pocket. The bivalent nature of the receptor binding of these compounds is believed to contribute to their D3 selectivity. In this study, we examined if such compounds might also be "bitopic" such that their aryl amide moieties act as allosteric modulators to further enhance the affinities of the full-length molecules for the receptor. First, we deconstructed several extended-length D3-selective ligands into fragments, termed "synthons", representing either orthosteric or secondary aryl amide pharmacophores and investigated their effects on D3 receptor binding and function. The orthosteric synthons were found to inhibit radioligand binding and to antagonize dopamine activation of the D3 receptor, albeit with lower affinities than the full-length compounds. Notably, the aryl amide-based synthons had no effect on the affinities or potencies of the orthosteric synthons, nor did they have any effect on receptor activation by dopamine. Additionally, pharmacological investigation of the full-length D3-selective antagonists revealed that these compounds interacted with the D3 receptor in a purely competitive manner. Our data further support that the 4-phenylpiperazine D3-selective antagonists are bivalent and that their enhanced affinity for the D3 receptor is due to binding at both the orthosteric site as well as a secondary binding pocket. Importantly, however, their interactions at the secondary site do not allosterically modulate their binding to the orthosteric site.
Keywords: Allosteric; Bitopic; Bivalent; D(3) receptor; Dopamine antagonists; Dopamine receptor.
Published by Elsevier B.V.
Conflict of interest statement
All authors report no conflicts of interest in this study.
Figures




References
-
- Banala AK, Levy BA, Khatri SS, Furman CA, Roof RA, Mishra Y, Griffin SA, Sibley DR, Luedtke RR, Newman AH. N-(3-fluoro-4-(4-(2-methoxy or 2,3-dichlorophenyl)piperazine-1-yl) butyl)arylcarboxamides as selective dopamine D3 receptor ligands: critical role of the carboxamide linker for D3 receptor selectivity. J Med Chem. 2011;54:3581–3594. - PMC - PubMed
-
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. - PubMed
-
- Boeckler F, Gmeiner P. The structural evolution of dopamine D3 receptor ligands: structure-activity relationships and selected neuropharmacological aspects. Pharmacol Ther. 2006;112:281–333. - PubMed
-
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

