Visualization of the type III secretion sorting platform of Shigella flexneri
- PMID: 25583506
- PMCID: PMC4313800
- DOI: 10.1073/pnas.1411610112
Visualization of the type III secretion sorting platform of Shigella flexneri
Abstract
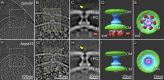
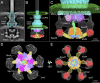
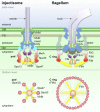
Bacterial type III secretion machines are widely used to inject virulence proteins into eukaryotic host cells. These secretion machines are evolutionarily related to bacterial flagella and consist of a large cytoplasmic complex, a transmembrane basal body, and an extracellular needle. The cytoplasmic complex forms a sorting platform essential for effector selection and needle assembly, but it remains largely uncharacterized. Here we use high-throughput cryoelectron tomography (cryo-ET) to visualize intact machines in a virulent Shigella flexneri strain genetically modified to produce minicells capable of interaction with host cells. A high-resolution in situ structure of the intact machine determined by subtomogram averaging reveals the cytoplasmic sorting platform, which consists of a central hub and six spokes, with a pod-like structure at the terminus of each spoke. Molecular modeling of wild-type and mutant machines allowed us to propose a model of the sorting platform in which the hub consists mainly of a hexamer of the Spa47 ATPase, whereas the MxiN protein comprises the spokes and the Spa33 protein forms the pods. Multiple contacts among those components are essential to align the Spa47 ATPase with the central channel of the MxiA protein export gate to form a unique nanomachine. The molecular architecture of the Shigella type III secretion machine and its sorting platform provide the structural foundation for further dissecting the mechanisms underlying type III secretion and pathogenesis and also highlight the major structural distinctions from bacterial flagella.
Keywords: cryo-electron tomography; injectisome; nanomachine; pathogen–host interaction; protein secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4(11):811–825. - PubMed
-
- Galán JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444(7119):567–573. - PubMed
-
- Kubori T, et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280(5363):602–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

