An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence
- PMID: 25583510
- PMCID: PMC4343097
- DOI: 10.1073/pnas.1416485112
An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence
Abstract
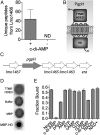
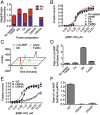
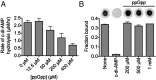
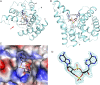
The nucleotide cyclic di-3',5'- adenosine monophosphate (c-di-AMP) was recently identified as an essential and widespread second messenger in bacterial signaling. Among c-di-AMP-producing bacteria, altered nucleotide levels result in several physiological defects and attenuated virulence. Thus, a detailed molecular understanding of c-di-AMP metabolism is of both fundamental and practical interest. Currently, c-di-AMP degradation is recognized solely among DHH-DHHA1 domain-containing phosphodiesterases. Using chemical proteomics, we identified the Listeria monocytogenes protein PgpH as a molecular target of c-di-AMP. Biochemical and structural studies revealed that the PgpH His-Asp (HD) domain bound c-di-AMP with high affinity and specifically hydrolyzed this nucleotide to 5'-pApA. PgpH hydrolysis activity was inhibited by ppGpp, indicating a cross-talk between c-di-AMP signaling and the stringent response. Genetic analyses supported coordinated regulation of c-di-AMP levels in and out of the host. Intriguingly, a L. monocytogenes mutant that lacks c-di-AMP phosphodiesterases exhibited elevated c-di-AMP levels, hyperinduced a host type-I IFN response, and was significantly attenuated for infection. Furthermore, PgpH homologs, which belong to the 7TMR-HD family, are widespread among hundreds of c-di-AMP synthesizing microorganisms. Thus, PgpH represents a broadly conserved class of c-di-AMP phosphodiesterase with possibly other physiological functions in this crucial signaling network.
Keywords: HD domain; Listeria monocytogenes; bacterial signal transduction; c-di-AMP; phosphodiesterase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

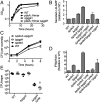
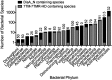
Comment in
-
Chemical proteomics reveals a second family of cyclic-di-AMP hydrolases.Proc Natl Acad Sci U S A. 2015 Feb 17;112(7):1921-2. doi: 10.1073/pnas.1500077112. Epub 2015 Jan 30. Proc Natl Acad Sci U S A. 2015. PMID: 25637595 Free PMC article. No abstract available.
References
-
- Kalia D, et al. Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chem Soc Rev. 2013;42(1):305–341. - PubMed
-
- Römling U. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal. 2008;1(33):pe39. - PubMed
-
- Corrigan RM, Gründling A. Cyclic di-AMP: Another second messenger enters the fray. Nat Rev Microbiol. 2013;11(8):513–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

