Importance of bacterial replication and alveolar macrophage-independent clearance mechanisms during early lung infection with Streptococcus pneumoniae
- PMID: 25583525
- PMCID: PMC4333471
- DOI: 10.1128/IAI.02788-14
Importance of bacterial replication and alveolar macrophage-independent clearance mechanisms during early lung infection with Streptococcus pneumoniae
Abstract
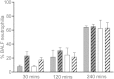
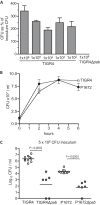
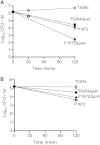
Although the importance of alveolar macrophages for host immunity during early Streptococcus pneumoniae lung infection is well established, the contribution and relative importance of other innate immunity mechanisms and of bacterial factors are less clear. We have used a murine model of S. pneumoniae early lung infection with wild-type, unencapsulated, and para-amino benzoic acid auxotroph mutant TIGR4 strains to assess the effects of inoculum size, bacterial replication, capsule, and alveolar macrophage-dependent and -independent clearance mechanisms on bacterial persistence within the lungs. Alveolar macrophage-dependent and -independent (calculated indirectly) clearance half-lives and bacterial replication doubling times were estimated using a mathematical model. In this model, after infection with a high-dose inoculum of encapsulated S. pneumoniae, alveolar macrophage-independent clearance mechanisms were dominant, with a clearance half-life of 24 min compared to 135 min for alveolar macrophage-dependent clearance. In addition, after a high-dose inoculum, successful lung infection required rapid bacterial replication, with an estimated S. pneumoniae doubling time of 16 min. The capsule had wide effects on early lung clearance mechanisms, with reduced half-lives of 14 min for alveolar macrophage-independent and 31 min for alveolar macrophage-dependent clearance of unencapsulated bacteria. In contrast, with a lower-dose inoculum, the bacterial doubling time increased to 56 min and the S. pneumoniae alveolar macrophage-dependent clearance half-life improved to 42 min and was largely unaffected by the capsule. These data demonstrate the large effects of bacterial factors (inoculum size, the capsule, and rapid replication) and alveolar macrophage-independent clearance mechanisms during early lung infection with S. pneumoniae.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Lee H-Y, Andalibi A, Webster P, Moon S-K, Teufert K, Kang S-H, Li J-D, Nagura M, Ganz T, Lim DJ. 2004. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis 4:12. doi:10.1186/1471-2334-4-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

