MyD88-dependent signaling drives host survival and early cytokine production during Histoplasma capsulatum infection
- PMID: 25583527
- PMCID: PMC4363404
- DOI: 10.1128/IAI.02619-14
MyD88-dependent signaling drives host survival and early cytokine production during Histoplasma capsulatum infection
Abstract
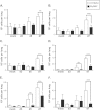
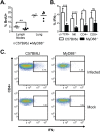
The ability of the innate immune system to trigger an adaptive T cell response is critical to resolution of infection with the fungal pathogen Histoplasma capsulatum. However, the signaling pathways and cell types involved in the recognition of and response to this respiratory pathogen remain poorly defined. Here, we show that MyD88, an adaptor protein vital to multiple innate immune pathways, is critically required for the host response to Histoplasma. MyD88-deficient (MyD88-/-) mice are unable to control the fungal burden and are more sensitive to Histoplasma infection than wild-type, Dectin-1-/-, or interleukin 1 receptor-deficient (IL-1R-/-) mice. We found that MyD88 is necessary for the production of key early inflammatory cytokines and the subsequent recruitment of inflammatory monocytes to the lung. In both our in vitro and ex vivo analyses, MyD88 was intrinsically required in dendritic cells and alveolar macrophages for initial cytokine production. Additionally, MyD88-deficient bone marrow-derived dendritic cells fail to efficiently control fungal growth when cocultured with primed splenic T cells. Surprisingly, mice that lack MyD88 only in dendritic cells and alveolar macrophages are competent for early cytokine production and normal survival, indicating the presence of compensatory and redundant MyD88 signaling in other cell types during infection. Ultimately, global MyD88 deficiency prevents proper T cell activation and gamma interferon (IFN-γ) production, which are critical for infection resolution. Collectively, this work reveals a central role for MyD88 in coordinating the innate and adaptive immune responses to infection with this ubiquitous fungal pathogen of humans.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

