A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects
- PMID: 25583879
- PMCID: PMC4352589
- DOI: 10.1124/jpet.114.220392
A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects
Abstract
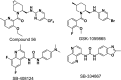
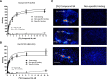
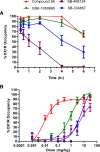
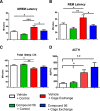
Orexins (OXs) are peptides produced by perifornical (PeF) and lateral hypothalamic neurons that exert a prominent role in arousal-related processes, including stress. A critical role for the orexin-1 receptor (OX1R) in complex emotional behavior is emerging, such as overactivation of the OX1R pathway being associated with panic or anxiety states. Here we characterize a brain-penetrant, selective, and high-affinity OX1R antagonist, compound 56 [N-({3-[(3-ethoxy-6-methylpyridin-2-yl)carbonyl]-3-azabicyclo[4.1.0]hept-4-yl}methyl)-5-(trifluoromethyl)pyrimidin-2-amine]. Ex vivo receptor binding studies demonstrated that, after subcutaneous administration, compound 56 crossed the blood-brain barrier and occupied OX1Rs in the rat brain at lower doses than standard OX1R antagonists GSK-1059865 [5-bromo-N-({1-[(3-fluoro-2-methoxyphenyl)carbonyl]-5-methylpiperidin-2-yl}methyl)pyridin-2-amine], SB-334867 [1-(2-methyl-1,3-benzoxazol-6-yl)-3-(1,5-naphthyridin-4-yl)urea], and SB-408124 [1-(6,8-difluoro-2-methylquinolin-4-yl)-3-[4-(dimethylamino)phenyl]urea]. Although compound 56 did not alter spontaneous sleep in rats and in wild-type mice, its administration in orexin-2 receptor knockout mice selectively promoted rapid eye movement sleep, demonstrating target engagement and specific OX1R blockade. In a rat model of psychological stress induced by cage exchange, the OX1R antagonist prevented the prolongation of sleep onset without affecting sleep duration. In a rat model of panic vulnerability (involving disinhibition of the PeF OX region) to threatening internal state changes (i.e., intravenous sodium lactate infusion), compound 56 attenuated sodium lactate-induced panic-like behaviors and cardiovascular responses without altering baseline locomotor or autonomic activity. In conclusion, OX1R antagonism represents a novel therapeutic strategy for the treatment of various psychiatric disorders associated with stress or hyperarousal states.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Abshire VM, Hankins KD, Roehr KE, DiMicco JA. (1988) Injection of L-allylglycine into the posterior hypothalamus in rats causes decreases in local GABA which correlate with increases in heart rate. Neuropharmacology 27:1171–1177. - PubMed
-
- Alvaro G, Amantini D, Castiglioni E, Di Fabio R and Pavone F (2010) inventors, Glaxo Group Limited UK, assignee. N-{[(1S,4S,6S)-3-(2-Pyridinylcarbonyl)-3-azabicyclo[4.1.0]hept-4-yl]methyl}-2-heteroarylamine derivatives as orexin receptor antagonists and their preparation and use for the treatment of diseases. WO2010063662A1
-
- Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, Soya H. (2007) Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci Res 57:462–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

