Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer
- PMID: 25584002
- PMCID: PMC4397277
- DOI: 10.1200/JCO.2014.57.4244
Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer
Abstract
Purpose: GVAX pancreas, granulocyte-macrophage colony-stimulating factor-secreting allogeneic pancreatic tumor cells, induces T-cell immunity to cancer antigens, including mesothelin. GVAX is administered with low-dose cyclophosphamide (Cy) to inhibit regulatory T cells. CRS-207, live-attenuated Listeria monocytogenes-expressing mesothelin, induces innate and adaptive immunity. On the basis of preclinical synergy, we tested prime/boost vaccination with GVAX and CRS-207 in pancreatic adenocarcinoma.
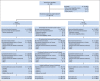
Patients and methods: Previously treated patients with metastatic pancreatic adenocarcinoma were randomly assigned at a ratio of 2:1 to two doses of Cy/GVAX followed by four doses of CRS-207 (arm A) or six doses of Cy/GVAX (arm B) every 3 weeks. Stable patients were offered additional courses. The primary end point was overall survival (OS) between arms. Secondary end points were safety and clinical response.
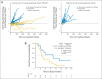
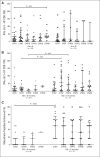
Results: A total of 90 patients were treated (arm A, n = 61; arm B, n = 29); 97% had received prior chemotherapy; 51% had received ≥ two regimens for metastatic disease. Mean number of doses (± standard deviation) administered in arms A and B were 5.5 ± 4.5 and 3.7 ± 2.2, respectively. The most frequent grade 3 to 4 related toxicities were transient fevers, lymphopenia, elevated liver enzymes, and fatigue. OS was 6.1 months in arm A versus 3.9 months in arm B (hazard ratio [HR], 0.59; P = .02). In a prespecified per-protocol analysis of patients who received at least three doses (two doses of Cy/GVAX plus one of CRS-207 or three of Cy/GVAX), OS was 9.7 versus 4.6 months (arm A v B; HR, 0.53; P = .02). Enhanced mesothelin-specific CD8 T-cell responses were associated with longer OS, regardless of treatment arm.
Conclusion: Heterologous prime/boost with Cy/GVAX and CRS-207 extended survival for patients with pancreatic cancer, with minimal toxicity.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures




Comment in
-
Precision immunology: the promise of immunotherapy for the treatment of cancer.J Clin Oncol. 2015 Apr 20;33(12):1315-7. doi: 10.1200/JCO.2014.59.6023. Epub 2015 Jan 20. J Clin Oncol. 2015. PMID: 25605852 No abstract available.
-
Pancreatic cancer: vaccine is safe and improves survival.Nat Rev Clin Oncol. 2015 Mar;12(3):128. doi: 10.1038/nrclinonc.2015.11. Epub 2015 Feb 3. Nat Rev Clin Oncol. 2015. PMID: 25645600 No abstract available.
References
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

