Transarterial chemoembolization in unresectable hepatocellular carcinoma with portal vein thrombosis: a perspective on survival
- PMID: 25584161
- PMCID: PMC4289489
- DOI: 10.5001/omj.2014.114
Transarterial chemoembolization in unresectable hepatocellular carcinoma with portal vein thrombosis: a perspective on survival
Abstract
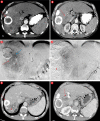
Objective: Portal vein thrombosis is considered a relative contraindication for transarterial chemoembolization (TACE) in hepatocellular carcinoma. The purpose of our study was to evaluate the efficacy of TACE treatment in patients with hepatocellular carcinoma with portal vein (PV) thrombosis.
Methods: From April 2011 to June 2013, 17 patients with unresectable hepatocellular carcinoma with PV thrombosis were studied. Patients were assessed for tumor response by imaging at regular intervals and the data compared with the baseline laboratory and imaging characteristics obtained before treatment. Univariate analysis was used to assess the treatments impact on patient survival. Survival analysis was performed using Kaplan-Meier estimations.
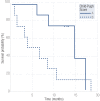
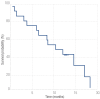
Results: Overall survival rates at three, six and 12 months were 82%, 71%, and 47%, respectively, with a median of 10 months. Patients in Child-Pugh class A had a median survival of 15 months compared to five months for those patients in Child-Pugh class B. The median survival period of patients responsive to treatment was 13 months while that of non-responders was five months. Patients with ascites at the time of presentation had median survival period of six months while those who did not had a median survival period of 13 months. In univariate analysis, response to chemoembolization (p<0.001), ascites (p<0.050) and Child-Pugh class at diagnosis (p<0.050) were found to be significant prognostic factors.
Conclusion: TACE is a promising procedure in unresectable hepatocellular carcinoma with PV thrombosis. Response to chemoembolization, ascites and Child-Pugh class were the most important determining factors of survival.
Keywords: Chemoembolization; Hepatocellular carcinoma; Portal vein; Survival Analysis; Thrombosis.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
