Therapeutic potential of gingival fibroblasts for cutaneous radiation syndrome: comparison to bone marrow-mesenchymal stem cell grafts
- PMID: 25584741
- PMCID: PMC4425223
- DOI: 10.1089/scd.2014.0486
Therapeutic potential of gingival fibroblasts for cutaneous radiation syndrome: comparison to bone marrow-mesenchymal stem cell grafts
Abstract
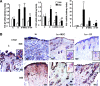
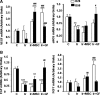
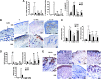
Mesenchymal stem cell (MSC) therapy has recently been investigated as a potential treatment for cutaneous radiation burns. We tested the hypothesis that injection of local gingival fibroblasts (GFs) would promote healing of radiation burn lesions and compared results with those for MSC transplantation. Human clinical- grade GFs or bone marrow-derived MSCs were intradermally injected into mice 21 days after local leg irradiation. Immunostaining and real-time PCR analysis were used to assess the effects of each treatment on extracellular matrix remodeling and inflammation in skin on days 28 and 50 postirradiation. GFs induced the early development of thick, fully regenerated epidermis, skin appendages, and hair follicles, earlier than MSCs did. The acceleration of wound healing by GFs involved rearrangement of the deposited collagen, modification of the Col/MMP/TIMP balance, and modulation of the expression and localization of tenascin-C and of the expression of growth factors (VEGF, EGF, and FGF7). As MSC treatment did, GF injection decreased the irradiation-induced inflammatory response and switched the differentiation of macrophages toward an M2-like phenotype, characterized by CD163(+) macrophage infiltration and strong expression of arginase-1. These findings indicate that GFs are an attractive target for regenerative medicine, for easier to collect, can grow in culture, and promote cutaneous wound healing in irradiation burn lesions.
Figures
References
-
- Peter RU. (2005). Cutaneous radiation syndrome in multi-organ failure. BJR (Suppl. 27):180–184
-
- Hill RP, Rodemann HP, Hendry JH, Roberts SA. and Anscher MS. (2001). Normal tissue radiobiology: from the laboratory to the clinic. Int J Radiat Oncol Biol Phys 49:353–365 - PubMed
-
- Wu Y, Wang J, Scott PG. and Tredget EE. (2007). Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen 15 (Suppl. 1):S18–S26 - PubMed
-
- Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, Bottollier-Depois JF, Chapel A, Ernou I, et al. (2007). New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med 2:785–794 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

