The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling
- PMID: 25584796
- PMCID: PMC4292982
- DOI: 10.1016/j.devcel.2014.11.016
The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling
Abstract
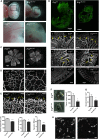
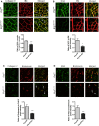
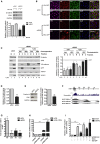
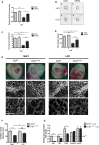
Blood vessel stability is essential for embryonic development; in the adult, many diseases are associated with loss of vascular integrity. The ETS transcription factor ERG drives expression of VE-cadherin and controls junctional integrity. We show that constitutive endothelial deletion of ERG (Erg(cEC-KO)) in mice causes embryonic lethality with vascular defects. Inducible endothelial deletion of ERG (Erg(iEC-KO)) results in defective physiological and pathological angiogenesis in the postnatal retina and tumors, with decreased vascular stability. ERG controls the Wnt/β-catenin pathway by promoting β-catenin stability, through signals mediated by VE-cadherin and the Wnt receptor Frizzled-4. Wnt signaling is decreased in ERG-deficient endothelial cells; activation of Wnt signaling with lithium chloride, which stabilizes β-catenin levels, corrects vascular defects in Erg(cEC-KO) embryos. Finally, overexpression of ERG in vivo reduces permeability and increases stability of VEGF-induced blood vessels. These data demonstrate that ERG is an essential regulator of angiogenesis and vascular stability through Wnt signaling.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Alastalo T.P., Li M., Perez Vde.J., Pham D., Sawada H., Wang J.K., Koskenvuo M., Wang L., Freeman B.A., Chang H.Y., Rabinovitch M. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J. Clin. Invest. 2011;121:3735–3746. - PMC - PubMed
-
- Baltzinger M., Mager-Heckel A.M., Remy P. Xl erg: expression pattern and overexpression during development plead for a role in endothelial cell differentiation. Dev. Dyn. 1999;216:420–433. - PubMed
-
- Birdsey G.M., Dryden N.H., Shah A.V., Hannah R., Hall M.D., Haskard D.O., Parsons M., Mason J.C., Zvelebil M., Gottgens B. The transcription factor Erg regulates expression of histone deacetylase 6 and multiple pathways involved in endothelial cell migration and angiogenesis. Blood. 2012;119:894–903. - PubMed
-
- Birney E., Stamatoyannopoulos J.A., Dutta A., Guigó R., Gingeras T.R., Margulies E.H., Weng Z., Snyder M., Dermitzakis E.T., Thurman R.E., ENCODE Project Consortium. NISC Comparative Sequencing Program. Baylor College of Medicine Human Genome Sequencing Center. Washington University Genome Sequencing Center. Broad Institute. Children’s Hospital Oakland Research Institute Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 12007/CRUK_/Cancer Research UK/United Kingdom
- PG/09/096/28114/BHF_/British Heart Foundation/United Kingdom
- PG/10/94/28651/BHF_/British Heart Foundation/United Kingdom
- 97922/WT_/Wellcome Trust/United Kingdom
- MC_PC_12009/MRC_/Medical Research Council/United Kingdom
- 18673/CRUK_/Cancer Research UK/United Kingdom
- G0901609/MRC_/Medical Research Council/United Kingdom
- 12765/CRUK_/Cancer Research UK/United Kingdom
- PG/09/096/BHF_/British Heart Foundation/United Kingdom
- 12-1068/AICR_/Worldwide Cancer Research/United Kingdom
- RG/11/17/29256/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

