Enhanced effects of isoflurane on the long QT syndrome 1-associated A341V mutant
- PMID: 25585005
- PMCID: PMC4366337
- DOI: 10.1097/ALN.0000000000000583
Enhanced effects of isoflurane on the long QT syndrome 1-associated A341V mutant
Abstract
Background: The impact of volatile anesthetics on patients with inherited long QT syndrome (LQTS) is not well understood. This is further complicated by the different genotypes underlying LQTS. No studies have reported on the direct effects of volatile anesthetics on specific LQTS-associated mutations. We investigated the effects of isoflurane on a common LQTS type 1 mutation, A341V, with an unusually severe phenotype.
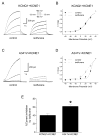
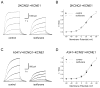
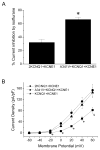
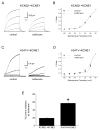
Methods: Whole cell potassium currents (IKs) were recorded from HEK293 and HL-1 cells transiently expressing/coexpressing wild-type KCNQ1 (α-subunit), mutant KCNQ1, wild-type KCNE1 (β-subunit), and fusion KCNQ1 + KCNE1. Current was monitored in the absence and presence of clinically relevant concentration of isoflurane (0.54 ± 0.05 mM, 1.14 vol %). Computer simulations determined the resulting impact on the cardiac action potential.
Results: Isoflurane had significantly greater inhibitory effect on A341V + KCNE1 (62.2 ± 3.4%, n = 8) than on wild-type KCNQ1 + KCNE1 (40.7 ± 4.5%; n = 9) in transfected HEK293 cells. Under heterozygous conditions, isoflurane inhibited A341V + KCNQ1 + KCNE1 by 65.2 ± 3.0% (n = 13) and wild-type KCNQ1 + KCNE1 (2:1 ratio) by 32.0 ± 4.5% (n = 11). A341V exerted a dominant negative effect on IKs. Similar differential effects of isoflurane were also observed in experiments using the cardiac HL-1 cells. Mutations of the neighboring F340 residue significantly attenuated the effects of isoflurane, and fusion proteins revealed the modulatory effect of KCNE1. Action potential simulations revealed a stimulation frequency-dependent effect of A341V.
Conclusions: The LQTS-associated A341V mutation rendered the IKs channel more sensitive to the inhibitory effects of isoflurane compared to wild-type IKs in transfected cell lines; F340 is a key residue for anesthetic action.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Partial restoration of the long QT syndrome associated KCNQ1 A341V mutant by the KCNE1 β-subunit.Biochim Biophys Acta. 2011 Dec;1810(12):1285-93. doi: 10.1016/j.bbagen.2011.07.018. Epub 2011 Aug 10. Biochim Biophys Acta. 2011. PMID: 21854832 Free PMC article.
-
Dominant-negative control of cAMP-dependent IKs upregulation in human long-QT syndrome type 1.Circ Res. 2012 Jan 20;110(2):211-9. doi: 10.1161/CIRCRESAHA.111.249482. Epub 2011 Nov 17. Circ Res. 2012. PMID: 22095730
-
The common long-QT syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds: toward a mutation-specific risk stratification.Circulation. 2007 Nov 20;116(21):2366-75. doi: 10.1161/CIRCULATIONAHA.107.726950. Epub 2007 Nov 5. Circulation. 2007. PMID: 17984373
-
A molecular mechanism for adrenergic-induced long QT syndrome.J Am Coll Cardiol. 2014 Mar 4;63(8):819-27. doi: 10.1016/j.jacc.2013.08.1648. Epub 2013 Oct 30. J Am Coll Cardiol. 2014. PMID: 24184248
-
KCNQ1 gene mutations and the respective genotype-phenotype correlations in the long QT syndrome.Med Sci Monit. 2002 Oct;8(10):RA240-8. Med Sci Monit. 2002. PMID: 12388934 Review.
Cited by
-
Overexpression of the Large-Conductance, Ca2+-Activated K+ (BK) Channel Shortens Action Potential Duration in HL-1 Cardiomyocytes.PLoS One. 2015 Jun 19;10(6):e0130588. doi: 10.1371/journal.pone.0130588. eCollection 2015. PLoS One. 2015. PMID: 26091273 Free PMC article.
References
-
- Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med. 2008;358:169–76. - PubMed
-
- Saenen JB, Vrints CJ. Molecular aspects of the congenital and acquired Long QT Syndrome: Clinical implications. J Mol Cell Cardiol. 2008;44:633–46. - PubMed
-
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. - PubMed
-
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. - PubMed
-
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

