A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality
- PMID: 25585275
- PMCID: PMC4359643
- DOI: 10.1016/j.bbmt.2015.01.001
A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality
Abstract
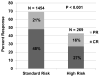
To develop a novel acute graft-versus-host disease (GVHD) risk score, we examined the GVHD clinical stage and grade of 1723 patients at the onset of treatment with systemic steroids. Using clinical grouping, descriptive statistics and recursive partitioning, we identified poorly responsive, high-risk (HR) acute GVHD by the number of involved organs and severity of GVHD at onset. The overall response (complete response/partial response) rate 28 days after initiation of steroid therapy for acute GVHD was lower in the 269 patients with HR-GVHD than in the 1454 patients with standard risk (SR)-GVHD (44% [95% confidence interval (CI) 38% to 50%] versus 68% [95% CI, 66% to 70%], P < .001). Patients with HR-GVHD were less likely to respond at day 28 (odds ratio [OR], .3; 95% CI, .2 to .4; P < .001) and had higher risks of mortality (relative risk, 2.1; 95% CI, 1.7 to 2.6; P < .001) and transplant-related mortality (relative risk, 2.5; 95% CI, 2.0% to 3.2%, P < .001) than patients with SR-GVHD. This refined definition of acute GVHD risk is a better predictor of response, survival, and transplant-related mortality than other published acute GVHD risk scores. Patients with HR-GVHD are candidates for studies investigating new treatment approaches. Likewise, patients with SR-GVHD are candidates for studies investigating less toxic therapy.
Keywords: Allogeneic hematopoietic cell transplantation; Grading systems; Graft-versus-host disease; Risk score; Survival; Transplant-related mortality.
Copyright © 2015 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–30. - PubMed
-
- MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94. - PubMed
-
- Martino R, Romero P, Subira M, et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry Bone Marrow Transplant. 1999;24:283–7. - PubMed
-
- Weisdorf DJ, Hurd D, Carter S, et al. Prospective grading of graft-versus-host disease after unrelated donor marrow transplantation: a grading algorithm versus blinded expert panel review. Biol Blood Marrow Transplant. 2003;9:512–8. - PubMed
-
- Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–500. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- U10 HL069294/HL/NHLBI NIH HHS/United States
- UG1 HL069286/HL/NHLBI NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- U10 HL069290/HL/NHLBI NIH HHS/United States
- U10 HL069330/HL/NHLBI NIH HHS/United States
- U10 HL069310/HL/NHLBI NIH HHS/United States
- U10HL069290/HL/NHLBI NIH HHS/United States
- U10 HL109526/HL/NHLBI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U10 HL069286/HL/NHLBI NIH HHS/United States
- U10 HL069249/HL/NHLBI NIH HHS/United States
- U10 HL069334/HL/NHLBI NIH HHS/United States
- U10 HL109137/HL/NHLBI NIH HHS/United States
- 2P01CA065493/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

