Glucose modulation induces reactive oxygen species and increases P-glycoprotein-mediated multidrug resistance to chemotherapeutics
- PMID: 25586174
- PMCID: PMC4409907
- DOI: 10.1111/bph.13079
Glucose modulation induces reactive oxygen species and increases P-glycoprotein-mediated multidrug resistance to chemotherapeutics
Abstract
Background and purpose: Cancer cells develop resistance to stress induced by chemotherapy. In tumours, a considerable glucose gradient exists, resulting in stress. Notably, hypoxia-inducible factor-1 (HIF-1) is a redox-sensitive transcription factor that regulates P-glycoprotein (Pgp), a crucial drug-efflux transporter involved in multidrug resistance (MDR). Here, we investigated how glucose levels regulate Pgp-mediated drug transport and resistance.
Experimental approach: Human tumour cells (KB31, KBV1, A549 and DMS-53) were incubated under glucose starvation to hyperglycaemic conditions. Flow cytometry assessed reactive oxygen species (ROS) generation and Pgp activity. HIF-1α, NF-κB and Pgp expression were assessed by reverse transcriptase-PCR and Western blotting. Fluorescence microscopy examined p65 distribution and a luciferase-reporter assay assessed HIF-1 promoter-binding activity. The effect of glucose-induced stress on Pgp-mediated drug resistance was examined after incubating cells with the chemotherapeutic and Pgp substrate, doxorubicin (DOX), and performing MTT assays validated by viable cell counts.
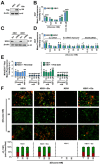
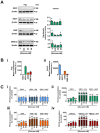
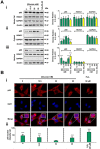
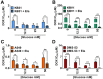
Key results: Changes in glucose levels markedly enhanced cellular ROS and conferred Pgp-mediated drug resistance. Low and high glucose levels increased (i) ROS generation via NADPH oxidase 4 and mitochondrial membrane destabilization; (ii) HIF-1 activity; (iii) nuclear translocation of the NF-κB p65 subunit; and (iv) HIF-1α mRNA and protein levels. Increased HIF-1α could also be due to decreased prolyl hydroxylase protein under these conditions. The HIF-1α target, Pgp, was up-regulated at low and high glucose levels, which led to lower cellular accumulation of Pgp substrate, rhodamine123, and greater resistance to DOX.
Conclusions and implications: As tumour cells become glucose-deprived or exposed to high glucose levels, this increases stress, leading to a more aggressive MDR phenotype via up-regulation of Pgp.
© 2015 The British Pharmacological Society.
Figures

References
-
- Akhtar N, Ahad A, Khar RK, Jaggi M, Aqil M, Iqbal Z, et al. The emerging role of P-glycoprotein inhibitors in drug delivery: a patent review. Expert Opin Ther Pat. 2011;21:561–576. - PubMed
-
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. - PubMed
-
- Annibaldi A, Widmann C. Glucose metabolism in cancer cells. Curr Opin Clin Nutr Metab Care. 2010;13:466–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

