A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study
- PMID: 25586468
- PMCID: PMC4483122
- DOI: 10.1093/neuonc/nou348
A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study
Abstract
Background: The epidermal growth factor receptor variant III deletion mutation, EGFRvIII, is expressed in ∼30% of primary glioblastoma and linked to poor long-term survival. Rindopepimut consists of the unique EGFRvIII peptide sequence conjugated to keyhole limpet hemocyanin. In previous phase II trials (ACTIVATE/ACT II), rindopepimut was well tolerated with robust EGFRvIII-specific immune responses and promising progression-free and overall survival. This multicenter, single-arm phase II clinical trial (ACT III) was performed to confirm these results.
Methods: Rindopepimut and standard adjuvant temozolomide chemotherapy were administered to 65 patients with newly diagnosed EGFRvIII-expressing (EGFRvIII+) glioblastoma after gross total resection and chemoradiation.
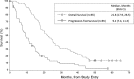
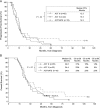
Results: Progression-free survival at 5.5 months (∼8.5 mo from diagnosis) was 66%. Relative to study entry, median overall survival was 21.8 months, and 36-month overall survival was 26%. Extended rindopepimut vaccination (up to 3.5+ years) was well tolerated. Grades 1-2 injection site reactions were frequent. Anti-EGFRvIII antibody titers increased ≥4-fold in 85% of patients, and increased with duration of treatment. EGFRvIII was eliminated in 4/6 (67%) tumor samples obtained after >3 months of therapy.
Conclusions: This study confirms, in a multicenter setting, the preliminary results seen in previous phase II trials of rindopepimut. A pivotal, double-blind, randomized, phase III trial ("ACT IV") is under way.
Keywords: ACT III; EGFRvIII; glioblastoma; glioma; rindopepimut.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Immunotherapy for glioblastoma: are we finally getting closer?Neuro Oncol. 2015 Jun;17(6):771-2. doi: 10.1093/neuonc/nov070. Epub 2015 May 10. Neuro Oncol. 2015. PMID: 25964313 Free PMC article. No abstract available.
References
-
- Huang HS, Nagane M, Klingbeil CK, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;2725:2927–2935. - PubMed
-
- Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;2516:2288–2294. - PubMed
-
- Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;6320:6962–6970. - PubMed
-
- Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;114:1462–1466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

