Genetic regulation of murine pituitary development
- PMID: 25587054
- PMCID: PMC4335376
- DOI: 10.1530/JME-14-0237
Genetic regulation of murine pituitary development
Abstract
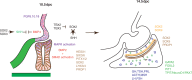
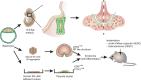
Significant progress has been made recently in unravelling the embryonic events leading to pituitary morphogenesis, both in vivo and in vitro. This includes dissection of the molecular mechanisms controlling patterning of the ventral diencephalon that regulate formation of the pituitary anlagen or Rathke's pouch. There is also a better characterisation of processes that underlie maintenance of pituitary progenitors, specification of endocrine lineages and the three-dimensional organisation of newly differentiated endocrine cells. Furthermore, a population of adult pituitary stem cells (SCs), originating from embryonic progenitors, have been described and shown to have not only regenerative potential, but also the capacity to induce tumour formation. Finally, the successful recapitulation in vitro of embryonic events leading to generation of endocrine cells from embryonic SCs, and their subsequent transplantation, represents exciting advances towards the use of regenerative medicine to treat endocrine deficits. In this review, an up-to-date description of pituitary morphogenesis will be provided and discussed with particular reference to pituitary SC studies.
Keywords: developmental factors; morphogenesis; pituitary; stem cell.
© 2015 The authors.
Figures

References
-
- Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13:433–445. doi: 10.1016/j.stem.2013.07.004. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

