Flow regulation of endothelin-1 production in the inner medullary collecting duct
- PMID: 25587122
- PMCID: PMC4360032
- DOI: 10.1152/ajprenal.00456.2014
Flow regulation of endothelin-1 production in the inner medullary collecting duct
Abstract
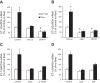
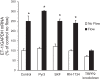
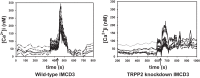
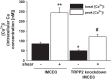
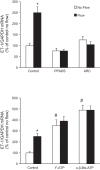
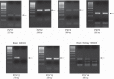
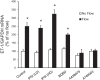
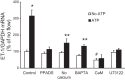
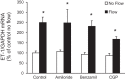
Collecting duct-derived endothelin (ET)-1 is an autocrine inhibitor of Na(+) and water reabsorption; its deficiency causes hypertension and water retention. Extracellular fluid volume expansion increases collecting duct ET-1, thereby promoting natriuresis and diuresis; however, how this coupling between volume expansion and collecting duct ET-1 occurs is incompletely understood. One possibility is that volume expansion increases tubular fluid flow. To investigate this, cultured IMCD3 cells were subjected to static or flow conditions. Exposure to a shear stress of 2 dyn/cm(2) for 2 h increased ET-1 mRNA content by ∼2.3-fold. Absence of perfusate Ca(2+), chelation of intracellular Ca(2+), or inhibition of Ca(2+) signaling (calmodulin, Ca(2+)/calmodulin-dependent kinase, calcineurin, PKC, or phospholipase C) prevented the flow response. Evaluation of possible flow-activated Ca(2+) entry pathways revealed no role for transient receptor potential (TRP)C3, TRPC6, and TRPV4; however, cells with TRPP2 (polycystin-2) knockdown had no ET-1 flow response. Flow increased intracellular Ca(2+) was blunted in TRPP2 knockdown cells. Nonspecific blockade of P2 receptors, as well as specific inhibition of P2X7 and P2Y2 receptors, prevented the ET-1 flow response. The ET-1 flow response was not affected by inhibition of either epithelial Na(+) channels or the mitochondrial Na(+)/Ca(2+) exchanger. Taken together, these findings provide evidence that in IMCD3 cells, flow, via polycystin-2 and P2 receptors, engages Ca(2+)-dependent signaling pathways that stimulate ET-1 synthesis.
Keywords: collecting duct; endothelin; flow; purinergic.
Figures
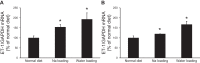
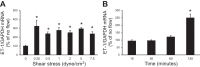


Comment in
-
Sorting out the paracrine kidney.Am J Physiol Renal Physiol. 2015 May 15;308(10):F1074-5. doi: 10.1152/ajprenal.00050.2015. Epub 2015 Feb 18. Am J Physiol Renal Physiol. 2015. PMID: 25694477 No abstract available.
References
-
- Abassi ZA, Klein H, Golomb E, Keiser HR. Regulation of the urinary excretion of endothelin in the rat. Am J Hypertens 6: 453–457, 1993. - PubMed
-
- Ando J, Komatsuda T, Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol 24: 871–877, 1988. - PubMed
-
- Barton M, Kohan DE. Endothelin antagonists in clinical trials: lessons learned. Contrib Nephrol 172: 255–260, 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

