Epigenetic regulation in human melanoma: past and future
- PMID: 25587943
- PMCID: PMC4622872
- DOI: 10.1080/15592294.2014.1003746
Epigenetic regulation in human melanoma: past and future
Abstract
The development and progression of melanoma have been attributed to independent or combined genetic and epigenetic events. There has been remarkable progress in understanding melanoma pathogenesis in terms of genetic alterations. However, recent studies have revealed a complex involvement of epigenetic mechanisms in the regulation of gene expression, including methylation, chromatin modification and remodeling, and the diverse activities of non-coding RNAs. The roles of gene methylation and miRNAs have been relatively well studied in melanoma, but other studies have shown that changes in chromatin status and in the differential expression of long non-coding RNAs can lead to altered regulation of key genes. Taken together, they affect the functioning of signaling pathways that influence each other, intersect, and form networks in which local perturbations disturb the activity of the whole system. Here, we focus on how epigenetic events intertwine with these pathways and contribute to the molecular pathogenesis of melanoma.
Keywords: 5hmC, 5-hydroxymethylcytosine; 5mC, 5-methylcytosine; ACE, angiotensin converting enzyme; ANCR, anti-differentiation non-coding RNA; ANRIL, antisense noncoding RNA in INK4 locus; ASK1, apoptosis signal-regulating kinase 1; ATRA, all-trans retinoic acid; BANCR, BRAF-activated non-coding RNA; BCL-2, B-cell lymphoma 2; BRAF, B-Raf proto-oncogene, serine/threonine kinase; BRG1, ATP-dependent helicase SMARCA4; CAF-1, chromatin assembly factor-1; CBX7, chromobox homolog 7; CCND1, cyclin D1; CD28, cluster of differentiation 28; CDK, cyclin-dependent kinase; CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; CHD8, chromodomain-helicase DNA-binding protein 8; CREB, cAMP response element-binding protein; CUDR, cancer upregulated drug resistant; Cdc6, cell division cycle 6; DNA methylation/demethylation; DNMT, DNA methyltransferase; EMT, epithelial-mesenchymal transition; ERK, extracellular signal-regulated kinase; EZH2, enhancer of zeste homolog 2; GPCRs, G-protein coupled receptors; GSK3a, glycogen synthase kinase 3 α; GWAS, genome-wide association study; HDAC, histone deacetylase; HOTAIR, HOX antisense intergenic RNA; IAP, inhibitor of apoptosis; IDH2, isocitrate dehydrogenase; IFN, interferon, interleukin 23; JNK, Jun N-terminal kinase; Jak/STAT, Janus kinase/signal transducer and activator of transcription; MAFG, v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog G; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; MAPK, mitogen-activated protein kinase; MC1R, melanocortin-1 receptor; MGMT, O6-methylguanine-DNA methyltransferase; MIF, macrophage migration inhibitory factor; MITF, microphthalmia-associated transcription factor; MRE, miRNA recognition element; MeCP2, methyl CpG binding protein 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOD, nucleotide-binding and oligomerization domain; PBX, pre-B-cell leukemia homeobox; PEDF, pigment epithelium derived factor; PI3K, phosphatidylinositol-4, 5-bisphosphate 3-kinase; PIB5PA, phosphatidylinositol-4, 5-biphosphate 5-phosphatase A; PKA, protein kinase A; PRC, polycomb repressor complex; PSF, PTB associated splicing factor; PTB, polypyrimidine tract-binding; PTEN, phosphatase and tensin homolog; RARB, retinoic acid receptor-β2; RASSF1A, Ras association domain family 1A; SETDB1, SET Domain, bifurcated 1; SPRY4, Sprouty 4; STAU1, Staufen1; SWI/SNF, SWItch/Sucrose Non-Fermentable; TCR, T-cell receptor; TET, ten eleven translocase; TGF β, transforming growth factor β; TINCR, tissue differentiation-inducing non-protein coding RNA; TOR, target of rapamycin; TP53, tumor protein 53; TRAF6, TNF receptor-associated factor 6; UCA1, urothelial carcinoma-associated 1; ceRNA, competitive endogenous RNAs; chromatin modification; chromatin remodeling; epigenetics; gene regulation; lncRNA, long ncRNA; melanoma; miRNA, micro RNA; ncRNA, non-coding RNA; ncRNAs; p14ARF, p14 alternative reading frame; p16INK4a, p16 inhibitor of CDK4; pRB, retinoblastoma protein; snoRNA, small nucleolar RNA; α-MSHm, α-melanocyte stimulating hormone.
Figures


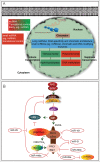
References
-
- Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893-917; PMID:21351269; http://dx.doi.org/10.1002/ijc.25516 - DOI - PubMed
-
- Uzdensky AB, Demyanenko SV, Bibov MY. Signal transduction in human cutaneous melanoma and target drugs. Curr Cancer Drug Targets 2013; 13:843-66; PMID:23675881; http://dx.doi.org/10.2174/1568009611313080004 - DOI - PubMed
-
- Pho LN, Leachman SA. Genetics of pigmentation and melanoma predisposition. G Ital Dermatol Venereol 2010; 145:37-45; PMID:20197744 - PubMed
-
- Sharpless E, Chin L. The INK4a/ARF locus and melanoma. Oncogene 2003; 22:3092-8; PMID:12789286; http://dx.doi.org/10.1038/sj.onc.1206461 - DOI - PubMed
-
- Griewank KG, Scolyer RA, Thompson JF, Flaherty KT, Schadendorf D, Murali R. Genetic alterations and personalized medicine in melanoma: progress and future prospects. J Natl Cancer Inst 2014; 106:djt435; PMID:24511108; http://dx.doi.org/10.1093/jnci/djt435 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
