Low-temperature polymorphic phase transition in a crystalline tripeptide L-Ala-L-Pro-Gly·H2O revealed by adiabatic calorimetry
- PMID: 25588051
- PMCID: PMC4442317
- DOI: 10.1021/jp508710g
Low-temperature polymorphic phase transition in a crystalline tripeptide L-Ala-L-Pro-Gly·H2O revealed by adiabatic calorimetry
Abstract
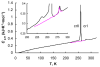
We demonstrate application of precise adiabatic vacuum calorimetry to observation of phase transition in the tripeptide L-alanyl-L-prolyl-glycine monohydrate (APG) from 6 to 320 K and report the standard thermodynamic properties of the tripeptide in the entire range. Thus, the heat capacity of APG was measured by adiabatic vacuum calorimetry in the above temperature range. The tripeptide exhibits a reversible first-order solid-to-solid phase transition characterized by strong thermal hysteresis. We report the standard thermodynamic characteristics of this transition and show that differential scanning calorimetry can reliably characterize the observed phase transition with <5 mg of the sample. Additionally, the standard entropy of formation from the elemental substances and the standard entropy of hypothetical reaction of synthesis from the amino acids at 298.15 K were calculated for the studied tripeptide.
Figures


Similar articles
-
Temperature-induced reversible first-order single crystal to single crystal phase transition in Boc-γ(4)(R)Val-Val-OH: interplay of enthalpy and entropy.J Phys Chem A. 2014 Oct 9;118(40):9568-74. doi: 10.1021/jp506874q. Epub 2014 Sep 23. J Phys Chem A. 2014. PMID: 25198546
-
Effect of the -Gly-3(S)-hydroxyprolyl-4(R)-hydroxyprolyl- tripeptide unit on the stability of collagen model peptides.FEBS J. 2008 Dec;275(23):5830-40. doi: 10.1111/j.1742-4658.2008.06704.x. FEBS J. 2008. PMID: 19021759
-
Structure and thermotropic properties of 1-stearoyl-2-acetyl-phosphatidylcholine bilayer membranes.Biophys J. 1994 May;66(5):1469-78. doi: 10.1016/S0006-3495(94)80937-3. Biophys J. 1994. PMID: 8061196 Free PMC article.
-
Theoretical Aspects of Differential Scanning Calorimetry as a Tool for the Studies of Equilibrium Thermodynamics in Pharmaceutical Solid Phase Transitions.AAPS PharmSciTech. 2016 Jun;17(3):572-7. doi: 10.1208/s12249-016-0530-2. Epub 2016 Apr 18. AAPS PharmSciTech. 2016. PMID: 27091667 Review.
-
Reversibility of enantiotropically related polymorphic transformations from a practical viewpoint: thermal analysis of kinetically reversible/irreversible polymorphic transformations.J Pharm Sci. 2007 May;96(5):982-9. doi: 10.1002/jps.20748. J Pharm Sci. 2007. PMID: 16937337 Review.
Cited by
-
Betulin-3,28-diphosphate. Physico-Chemical Properties and In Vitro Biological Activity Experiments.Molecules. 2018 May 14;23(5):1175. doi: 10.3390/molecules23051175. Molecules. 2018. PMID: 29757999 Free PMC article.
-
Peptide and Protein Dynamics and Low-Temperature/DNP Magic Angle Spinning NMR.J Phys Chem B. 2017 May 18;121(19):4997-5006. doi: 10.1021/acs.jpcb.7b02066. Epub 2017 May 10. J Phys Chem B. 2017. PMID: 28437077 Free PMC article.
References
-
- Jansson H, Bergman R, Swenson J. Role of Solvent for the Dynamics and the Glass Transition of Proteins. J. Phys. Chem. B. 2011;115:4099–4109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

