Herpes simplex virus 1 Us3 deletion mutant is infective despite impaired capsid translocation to the cytoplasm
- PMID: 25588052
- PMCID: PMC4306828
- DOI: 10.3390/v7010052
Herpes simplex virus 1 Us3 deletion mutant is infective despite impaired capsid translocation to the cytoplasm
Abstract
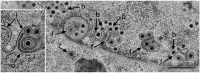
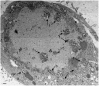
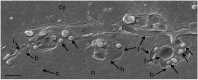
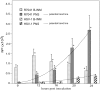
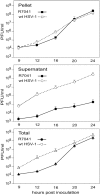
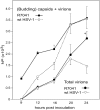
Herpes simplex virus 1 (HSV-1) capsids are assembled in the nucleus bud at the inner nuclear membrane into the perinuclear space, acquiring envelope and tegument. In theory, these virions are de-enveloped by fusion of the envelope with the outer nuclear membrane and re-enveloped by Golgi membranes to become infective. Us3 enables the nucleus to cytoplasm capsid translocation. Nevertheless, Us3 is not essential for the production of infective progeny viruses. Determination of phenotype distribution by quantitative electron microscopy, and calculation per mean nuclear or cell volume revealed the following: (i) The number of R7041(∆US3) capsids budding at the inner nuclear membrane was significantly higher than that of wild type HSV-1; (ii) The mean number of R7041(∆US3) virions per mean cell volume was 2726, that of HSV-1 virions 1460 by 24 h post inoculation; (iii) 98% of R7041(∆US3) virions were in the perinuclear space; (iv) The number of R7041(∆US3) capsids in the cytoplasm, including those budding at Golgi membranes, was significantly reduced. Cell associated R7041(∆US3) yields were 2.37×10(8) and HSV-1 yields 1.57×10(8) PFU/mL by 24 h post inoculation. We thus conclude that R7041(∆US3) virions, which acquire envelope and tegument by budding at the inner nuclear membrane into the perinuclear space, are infective.
Figures

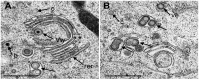

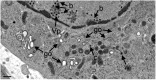

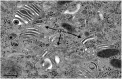
Similar articles
-
Nuclear envelope impairment is facilitated by the herpes simplex virus 1 Us3 kinase.F1000Res. 2019 Feb 18;8:198. doi: 10.12688/f1000research.17802.1. eCollection 2019. F1000Res. 2019. PMID: 31249678 Free PMC article.
-
The herpes simplex virus 1 Us3 kinase is involved in assembly of membranes needed for viral envelopment and in distribution of glycoprotein K.F1000Res. 2019 May 23;8:727. doi: 10.12688/f1000research.19194.1. eCollection 2019. F1000Res. 2019. PMID: 31448105 Free PMC article.
-
The Primary Enveloped Virion of Herpes Simplex Virus 1: Its Role in Nuclear Egress.mBio. 2017 Jun 13;8(3):e00825-17. doi: 10.1128/mBio.00825-17. mBio. 2017. PMID: 28611252 Free PMC article.
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
-
Virus Assembly and Egress of HSV.Adv Exp Med Biol. 2018;1045:23-44. doi: 10.1007/978-981-10-7230-7_2. Adv Exp Med Biol. 2018. PMID: 29896661 Review.
Cited by
-
Nuclear envelope impairment is facilitated by the herpes simplex virus 1 Us3 kinase.F1000Res. 2019 Feb 18;8:198. doi: 10.12688/f1000research.17802.1. eCollection 2019. F1000Res. 2019. PMID: 31249678 Free PMC article.
-
Molecular plasticity of herpesvirus nuclear egress analysed in situ.Nat Microbiol. 2024 Jul;9(7):1842-1855. doi: 10.1038/s41564-024-01716-8. Epub 2024 Jun 25. Nat Microbiol. 2024. PMID: 38918469 Free PMC article.
-
The mutated tegument protein UL7 attenuates the virulence of herpes simplex virus 1 by reducing the modulation of α-4 gene transcription.Virol J. 2016 Sep 13;13(1):152. doi: 10.1186/s12985-016-0600-9. Virol J. 2016. PMID: 27618986 Free PMC article.
-
Experimental Dissection of the Lytic Replication Cycles of Herpes Simplex Viruses in vitro.Front Microbiol. 2018 Oct 11;9:2406. doi: 10.3389/fmicb.2018.02406. eCollection 2018. Front Microbiol. 2018. PMID: 30386309 Free PMC article. Review.
-
Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home.Front Microbiol. 2020 May 7;11:733. doi: 10.3389/fmicb.2020.00733. eCollection 2020. Front Microbiol. 2020. PMID: 32457704 Free PMC article. Review.
References
-
- Roizman B., Knipe D.M., Whitley R.J. Herpes simplex viruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Wolters Kluver/Lipincott Wiliams & Wilkins; Philadelphia, PA, USA: 2014. pp. 1823–1897.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

