Differences in the distribution, phenotype and gene expression of subretinal microglia/macrophages in C57BL/6N (Crb1 rd8/rd8) versus C57BL6/J (Crb1 wt/wt) mice
- PMID: 25588310
- PMCID: PMC4305240
- DOI: 10.1186/s12974-014-0221-4
Differences in the distribution, phenotype and gene expression of subretinal microglia/macrophages in C57BL/6N (Crb1 rd8/rd8) versus C57BL6/J (Crb1 wt/wt) mice
Abstract
Background: Microglia/macrophages (MG/MΦ) are found in the subretinal space in both mice and humans. Our goal was to study the spatial and temporal distribution, the phenotype, and gene expression of subretinal MG/MΦ in mice with normal retinas and compare them to mice with known retinal pathology.
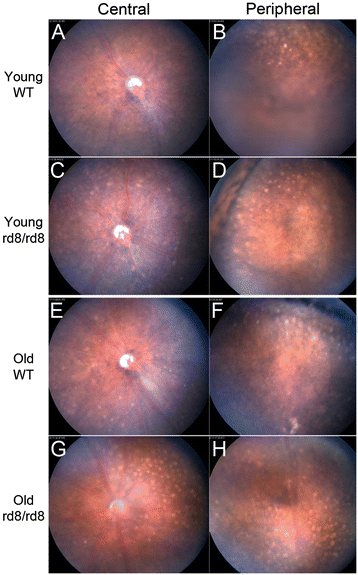
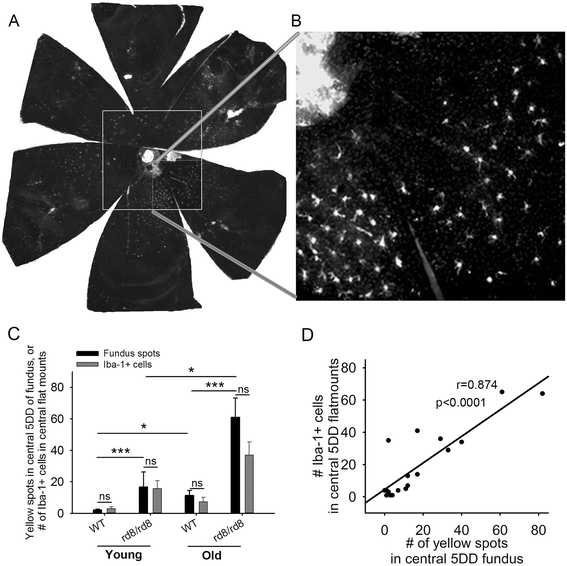
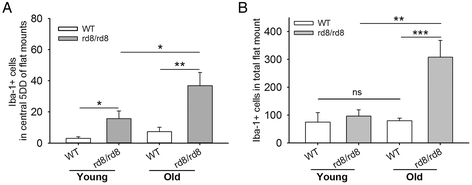
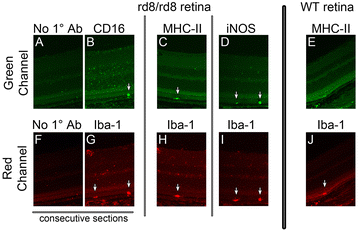
Methods: We studied C57BL/6 mice with (C57BL/6N), or without (C57BL/6J) the rd8 mutation in the Crb1 gene (which, in the presence of yet unidentified permissive/modifying genes, leads to a retinal degeneration), and documented their fundus appearance and the change with aging. Immunostaining of retinal pigment epithelium (RPE) flat mounts was done for 1) Ionized calcium binding adaptor (Iba)-1, 2) FcγIII/II Receptor (CD16/CD32, abbreviated as CD16), and 3) Macrophage mannose receptor (MMR). Reverse-transcription quantitative PCR (RT-qPCR) was done for genes involved in oxidative stress, complement activation and inflammation.
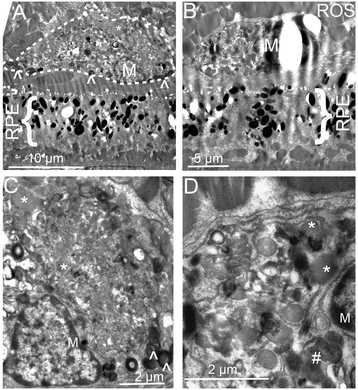
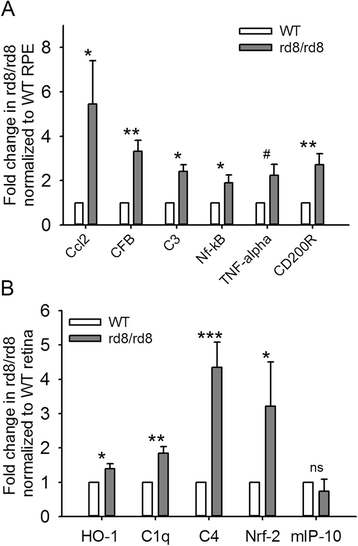
Results: The number of yellow fundus spots correlated highly with subretinal Iba-1+ cells. The total number of subretinal MG/MΦ increased with age in the rd8 mutant mice, but not in the wild-type (WT) mice. There was a centripetal shift in the distribution of the subretinal MG/MΦ with age. Old rd8 mutant mice had a greater number of CD16+ MG/MΦ. CD16+ cells had morphological signs of activation, and this was most prominent in old rd8 mutant mice (P < 1 × 10(-8) versus old WT mice). Subretinal MG/MΦ in rd8 mutant mice also expressed iNOS and MHC-II, and had ultrastructural signs of activation. Finally, rd8 mutant mouse RPE/ MG/MΦ RNA isolates showed an upregulation of Ccl2, CFB, C3, NF-kβ, CD200R and TNF-alpha. The retinas of rd8 mutant mice showed upregulation of HO-1, C1q, C4, and Nrf-2.
Conclusions: When compared to C57BL/6J mice, C57BL/6N mice demonstrate increased accumulation of subretinal MG/MΦ, displaying phenotypical, morphological, and gene-expression characteristics consistent with a pro-inflammatory shift. These changes become more prominent with aging and are likely due to the combination of the rd8 mutation and yet unidentified permissive/modulatory genes in the C57BL/6N mice. In contrast, aging leads to a scavenging phenotype in the C57BL/6J subretinal microglia/macrophages.
Figures


References
-
- Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration. The involvement of giant cells in atrophy of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1986;27:364–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

