Phloem as capacitor: radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma
- PMID: 25588734
- PMCID: PMC4348778
- DOI: 10.1104/pp.114.254581
Phloem as capacitor: radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma
Abstract
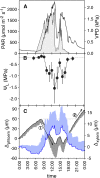
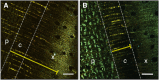
The transfer of water from phloem into xylem is thought to mitigate increasing hydraulic tension in the vascular system of trees during the diel cycle of transpiration. Although a putative plant function, to date there is no direct evidence of such water transfer or the contributing pathways. Here, we trace the radial flow of water from the phloem into the xylem and investigate its diel variation. Introducing a fluorescent dye (0.1% [w/w] fluorescein) into the phloem water of the tree species Eucalyptus saligna allowed localization of the dye in phloem and xylem tissues using confocal laser scanning microscopy. Our results show that the majority of water transferred between the two tissues is facilitated via the symplast of horizontal ray parenchyma cells. The method also permitted assessment of the radial transfer of water during the diel cycle, where changes in water potential gradients between phloem and xylem determine the extent and direction of radial transfer. When injected during the morning, when xylem water potential rapidly declined, fluorescein was translocated, on average, farther into mature xylem (447 ± 188 µm) compared with nighttime, when xylem water potential was close to zero (155 ± 42 µm). These findings provide empirical evidence to support theoretical predictions of the role of phloem-xylem water transfer in the hydraulic functioning of plants. This method enables investigation of the role of phloem tissue as a dynamic capacitor for water storage and transfer and its contribution toward the maintenance of the functional integrity of xylem in trees.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Barnard DM, Meinzer FC, Lachenbruch B, McCulloh KA, Johnson DM, Woodruff DR (2011) Climate-related trends in sapwood biophysical properties in two conifers: avoidance of hydraulic dysfunction through coordinated adjustments in xylem efficiency, safety and capacitance. Plant Cell Environ 34: 643–654 - PubMed
-
- Biddulph O, Markle J (1944) Translocation of radio-phosphorus in the phloem of the cotton plant. Am J Bot 31: 65–70
-
- Canny MJ, McCully ME (1986) Locating water-soluble vital stains in plant tissues by freeze-substitution and resin-embedding. J Microsc 142: 63–70
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

