Translational horizons in the tumor microenvironment: harnessing breakthroughs and targeting cures
- PMID: 25588753
- PMCID: PMC4374701
- DOI: 10.1002/med.21338
Translational horizons in the tumor microenvironment: harnessing breakthroughs and targeting cures
Abstract
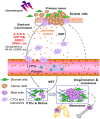
Chemotherapy and targeted therapy have opened new avenues in clinical oncology. However, there is a lack of response in a substantial percentage of cancer patients and diseases frequently relapse in those who even initially respond. Resistance is, at present, the major barrier to conquering cancer, the most lethal age-related pathology. Identification of mechanisms underlying resistance and development of effective strategies to circumvent treatment pitfalls thereby improving clinical outcomes remain overarching tasks for scientists and clinicians. Growing bodies of data indicate that stromal cells within the genetically stable but metabolically dynamic tumor microenvironment confer acquired resistance against anticancer therapies. Further, treatment itself activates the microenvironment by damaging a large population of benign cells, which can drastically exacerbate disease conditions in a cell nonautonomous manner, and such off-target effects should be well taken into account when establishing future therapeutic rationale. In this review, we highlight relevant biological mechanisms through which the tumor microenvironment drives development of resistance. We discuss some unsolved issues related to the preclinical and clinical trial paradigms that need to be carefully devised, and provide implications for personalized medicine. In the long run, an insightful and accurate understanding of the intricate signaling networks of the tumor microenvironment in pathological settings will guide the design of new clinical interventions particularly combinatorial therapies, and it might help overcome, or at least prevent, the onset of acquired resistance.
Keywords: acquired resistance; cancer therapy; clinical intervention; translational medicine; tumor microenvironment.
© 2015 Wiley Periodicals, Inc.
Figures



References
-
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. - PubMed
-
- Dubois C, Vanden Abeele F, Lehen'kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26(1):19–32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

