Enrichment of human hematopoietic stem/progenitor cells facilitates transduction for stem cell gene therapy
- PMID: 25588820
- PMCID: PMC5074681
- DOI: 10.1002/stem.1957
Enrichment of human hematopoietic stem/progenitor cells facilitates transduction for stem cell gene therapy
Abstract
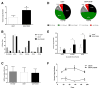
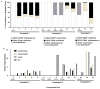
Autologous hematopoietic stem cell (HSC) gene therapy for sickle cell disease has the potential to treat this illness without the major immunological complications associated with allogeneic transplantation. However, transduction efficiency by β-globin lentiviral vectors using CD34-enriched cell populations is suboptimal and large vector production batches may be needed for clinical trials. Transducing a cell population more enriched for HSC could greatly reduce vector needs and, potentially, increase transduction efficiency. CD34(+) /CD38(-) cells, comprising ∼1%-3% of all CD34(+) cells, were isolated from healthy cord blood CD34(+) cells by fluorescence-activated cell sorting and transduced with a lentiviral vector expressing an antisickling form of beta-globin (CCL-β(AS3) -FB). Isolated CD34(+) /CD38(-) cells were able to generate progeny over an extended period of long-term culture (LTC) compared to the CD34(+) cells and required up to 40-fold less vector for transduction compared to bulk CD34(+) preparations containing an equivalent number of CD34(+) /CD38(-) cells. Transduction of isolated CD34(+) /CD38(-) cells was comparable to CD34(+) cells measured by quantitative PCR at day 14 with reduced vector needs, and average vector copy/cell remained higher over time for LTC initiated from CD34(+) /38(-) cells. Following in vitro erythroid differentiation, HBBAS3 mRNA expression was similar in cultures derived from CD34(+) /CD38(-) cells or unfractionated CD34(+) cells. In vivo studies showed equivalent engraftment of transduced CD34(+) /CD38(-) cells when transplanted in competition with 100-fold more CD34(+) /CD38(+) cells. This work provides initial evidence for the beneficial effects from isolating human CD34(+) /CD38(-) cells to use significantly less vector and potentially improve transduction for HSC gene therapy.
Keywords: Gene therapy; Hematopoietic stem cells; Lentiviral vector; Stem cell enrichment.
© 2015 AlphaMed Press.
Conflict of interest statement
of Potential Conflicts of Interest The authors indicate no potential conflicts of interest.
Figures
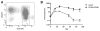

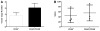

References
-
- Kohn DB, Pai SY, Sadelain M. Gene therapy through autologous transplantation of gene-modified hematopoietic stem cells. Biol Blood Marrow Transplant. 2013;19(suppl 1):S64–S69. - PubMed
-
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

