S1PR1 Tyr143 phosphorylation downregulates endothelial cell surface S1PR1 expression and responsiveness
- PMID: 25588843
- PMCID: PMC4342577
- DOI: 10.1242/jcs.154476
S1PR1 Tyr143 phosphorylation downregulates endothelial cell surface S1PR1 expression and responsiveness
Abstract
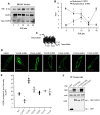
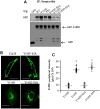
Activation of sphingosine-1-phosphate receptor 1 (S1PR1) plays a key role in repairing endothelial barrier function. We addressed the role of phosphorylation of the three intracellular tyrosine residues of S1PR1 in endothelial cells in regulating the receptor responsiveness and endothelial barrier function regulated by sphingosine 1-phosphate (S1P)-mediated activation of S1PR1. We demonstrated that phosphorylation of only Y143 site was required for S1PR1 internalization in response to S1P. Maximal S1PR1 internalization was seen in 20 min but S1PR1 returned to the cell surface within 1 h accompanied by Y143-dephosphorylation. Cell surface S1PR1 loss paralleled defective endothelial barrier enhancement induced by S1P. Expression of phospho-defective (Y143F) or phospho-mimicking (Y143D) mutants, respectively, failed to internalize or showed unusually high receptor internalization, consistent with the requirement of Y143 in regulating cell surface S1PR1 expression. Phosphorylation of the five S1PR1 C-terminal serine residues did not affect the role of Y143 phosphorylation in signaling S1PR1 internalization. Thus, rapid reduction of endothelial cell surface expression of S1PR1 subsequent to Y143 phosphorylation is a crucial mechanism of modulating S1PR1 signaling, and hence the endothelial barrier repair function of S1P.
Keywords: Adherens junctions; Endothelial barrier function; Endothelium; Serine phosphorylation; Sphingosine-1-phosphate receptor-1; Tyrosine phosphorylation.
© 2015. Published by The Company of Biologists Ltd.
Figures



References
-
- Bergelin N., Löf C., Balthasar S., Kalhori V., Törnquist K. (2010). S1P1 and VEGFR-2 form a signaling complex with extracellularly regulated kinase 1/2 and protein kinase C-alpha regulating ML-1 thyroid carcinoma cell migration. Endocrinology 151, 2994–3005. - PubMed
-
- Chen R. H., Derynck R. (1994). Homomeric interactions between type II transforming growth factor-beta receptors. J. Biol. Chem. 269, 22868–22874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

