Cytotoxic mediators in paradoxical HIV-tuberculosis immune reconstitution inflammatory syndrome
- PMID: 25589068
- PMCID: PMC4319311
- DOI: 10.4049/jimmunol.1402105
Cytotoxic mediators in paradoxical HIV-tuberculosis immune reconstitution inflammatory syndrome
Abstract
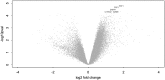
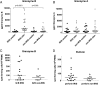
Tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) frequently complicates combined antiretroviral therapy and antituberculosis therapy in HIV-1-coinfected tuberculosis patients. The immunopathological mechanisms underlying TB-IRIS are incompletely defined, and improved understanding is required to derive new treatments and to reduce associated morbidity and mortality. We performed longitudinal and cross-sectional analyses of human PBMCs from paradoxical TB-IRIS patients and non-IRIS controls (HIV-TB-coinfected patients commencing antiretroviral therapy who did not develop TB-IRIS). Freshly isolated PBMC stimulated with heat-killed Mycobacterium tuberculosis H37Rv (hkH37Rv) were used for IFN-γ ELISPOT and RNA extraction. Stored RNA was used for microarray and RT-PCR, whereas corresponding stored culture supernatants were used for ELISA. Stored PBMC were used for perforin and granzyme B ELISPOT and flow cytometry. There were significantly increased IFN-γ responses to hkH37Rv in TB-IRIS, compared with non-IRIS PBMC (p = 0.035). Microarray analysis of hkH37Rv-stimulated PBMC indicated that perforin 1 was the most significantly upregulated gene, with granzyme B among the top five (log2 fold difference 3.587 and 2.828, respectively), in TB-IRIS. Downstream experiments using RT-PCR, ELISA, and ELISPOT confirmed the increased expression and secretion of perforin and granzyme B. Moreover, granzyme B secretion reduced in PBMC from TB-IRIS patients during corticosteroid treatment. Invariant NKT cell (CD3(+)Vα24(+)) proportions were higher in TB-IRIS patients (p = 0.004) and were a source of perforin. Our data implicate the granule exocytosis pathway in TB-IRIS pathophysiology. Further understanding of the immunopathogenesis of this condition will facilitate development of specific diagnostic and improved therapeutic options.
Copyright © 2015 The Authors.
Figures




References
-
- Chaisson R. E., Martinson N. A. 2008. Tuberculosis in Africa—combating an HIV-driven crisis. N. Engl. J. Med. 358: 1089–1092. - PubMed
-
- Schutz C., Meintjes G., Almajid F., Wilkinson R. J., Pozniak A. 2010. Clinical management of tuberculosis and HIV-1 co-infection. Eur. Respir. J. 36: 1460–1481. - PubMed
-
- Meintjes G., Lawn S. D., Scano F., Maartens G., French M. A., Worodria W., Elliott J. H., Murdoch D., Wilkinson R. J., Seyler C., et al. International Network for the Study of HIV-Associated IRIS 2008. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect. Dis. 8: 516–523. - PMC - PubMed
-
- Müller M., Wandel S., Colebunders R., Attia S., Furrer H., Egger M., IeDEA Southern and Central Africa 2010. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect. Dis. 10: 251–261. - PMC - PubMed
-
- Narendran G., Andrade B. B., Porter B. O., Chandrasekhar C., Venkatesan P., Menon P. A., Subramanian S., Anbalagan S., Bhavani K. P., Sekar S., et al. 2013. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 8: e63541. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

