Transcript profiling of different types of multiple sclerosis lesions yields FGF1 as a promoter of remyelination
- PMID: 25589163
- PMCID: PMC4359505
- DOI: 10.1186/s40478-014-0168-9
Transcript profiling of different types of multiple sclerosis lesions yields FGF1 as a promoter of remyelination
Abstract
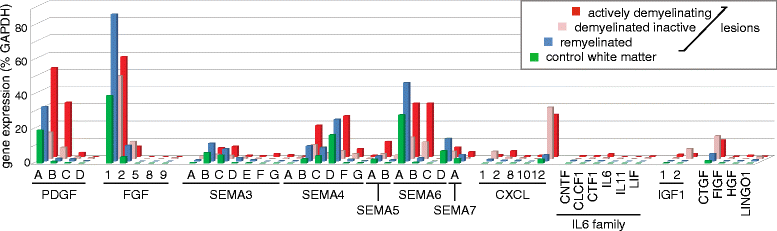
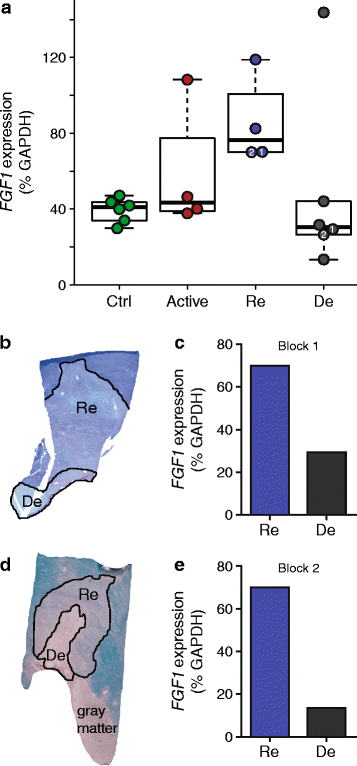
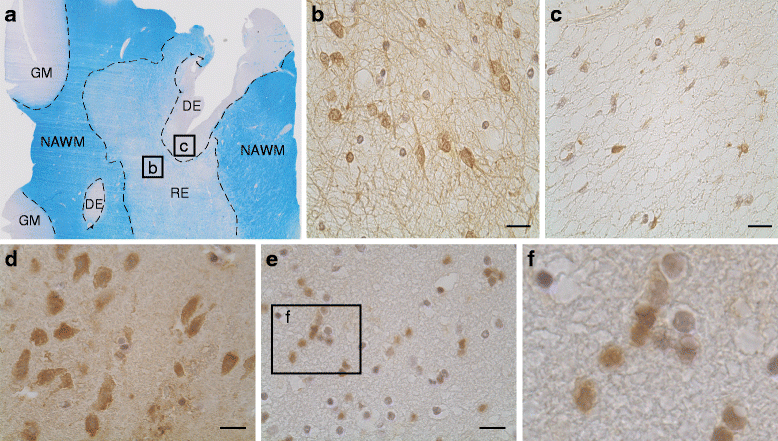
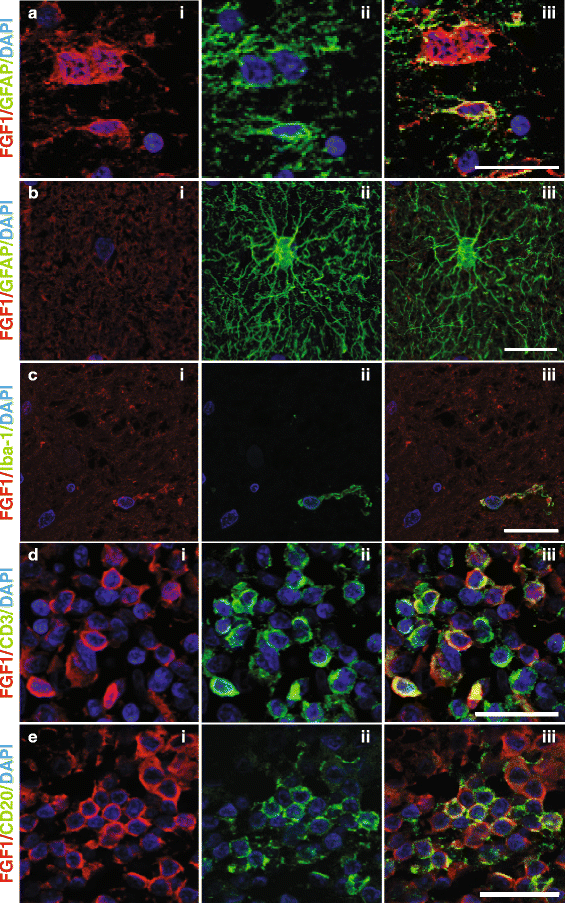
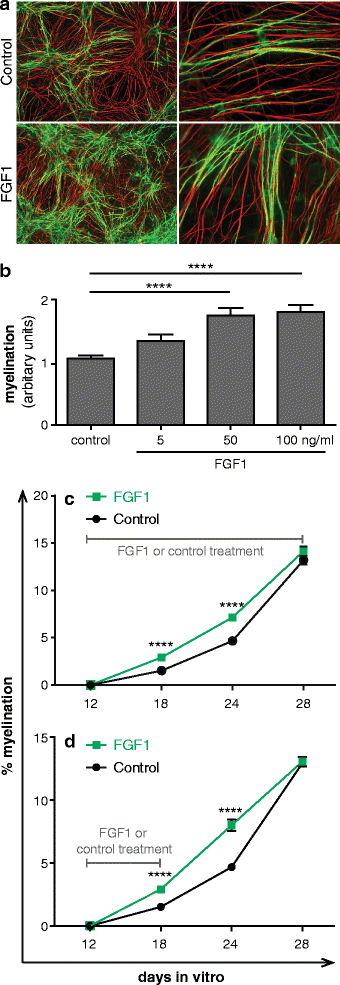
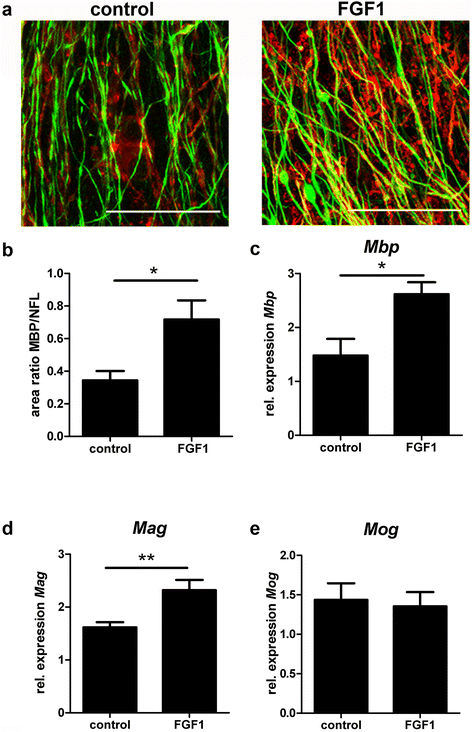
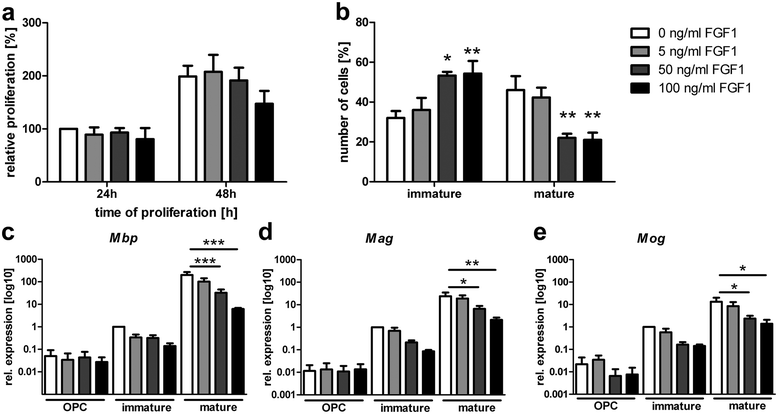
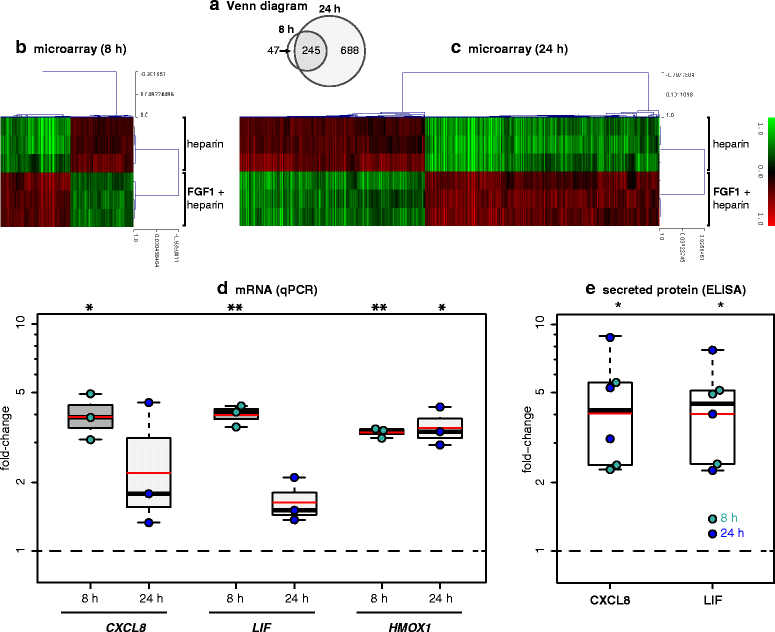
Chronic demyelination is a pathological hallmark of multiple sclerosis (MS). Only a minority of MS lesions remyelinates completely. Enhancing remyelination is, therefore, a major aim of future MS therapies. Here we took a novel approach to identify factors that may inhibit or support endogenous remyelination in MS. We dissected remyelinated, demyelinated active, and demyelinated inactive white matter MS lesions, and compared transcript levels of myelination and inflammation-related genes using quantitative PCR on customized TaqMan Low Density Arrays. In remyelinated lesions, fibroblast growth factor (FGF) 1 was the most abundant of all analyzed myelination-regulating factors, showed a trend towards higher expression as compared to demyelinated lesions and was significantly higher than in control white matter. Two MS tissue blocks comprised lesions with adjacent de- and remyelinated areas and FGF1 expression was higher in the remyelinated rim compared to the demyelinated lesion core. In functional experiments, FGF1 accelerated developmental myelination in dissociated mixed cultures and promoted remyelination in slice cultures, whereas it decelerated differentiation of purified primary oligodendrocytes, suggesting that promotion of remyelination by FGF1 is based on an indirect mechanism. The analysis of human astrocyte responses to FGF1 by genome wide expression profiling showed that FGF1 induced the expression of the chemokine CXCL8 and leukemia inhibitory factor, two factors implicated in recruitment of oligodendrocytes and promotion of remyelination. Together, this study presents a transcript profiling of remyelinated MS lesions and identified FGF1 as a promoter of remyelination. Modulation of FGF family members might improve myelin repair in MS.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

