Neural control of the lower urinary tract
- PMID: 25589273
- PMCID: PMC4480926
- DOI: 10.1002/cphy.c130056
Neural control of the lower urinary tract
Abstract
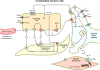
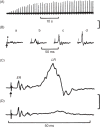
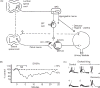
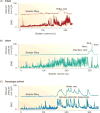
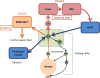
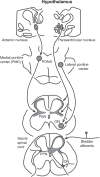
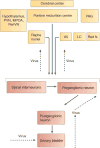
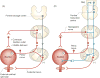
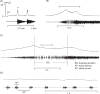
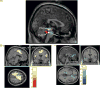
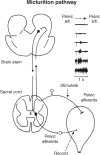
This article summarizes anatomical, neurophysiological, pharmacological, and brain imaging studies in humans and animals that have provided insights into the neural circuitry and neurotransmitter mechanisms controlling the lower urinary tract. The functions of the lower urinary tract to store and periodically eliminate urine are regulated by a complex neural control system in the brain, spinal cord, and peripheral autonomic ganglia that coordinates the activity of smooth and striated muscles of the bladder and urethral outlet. The neural control of micturition is organized as a hierarchical system in which spinal storage mechanisms are in turn regulated by circuitry in the rostral brain stem that initiates reflex voiding. Input from the forebrain triggers voluntary voiding by modulating the brain stem circuitry. Many neural circuits controlling the lower urinary tract exhibit switch-like patterns of activity that turn on and off in an all-or-none manner. The major component of the micturition switching circuit is a spinobulbospinal parasympathetic reflex pathway that has essential connections in the periaqueductal gray and pontine micturition center. A computer model of this circuit that mimics the switching functions of the bladder and urethra at the onset of micturition is described. Micturition occurs involuntarily in infants and young children until the age of 3 to 5 years, after which it is regulated voluntarily. Diseases or injuries of the nervous system in adults can cause the re-emergence of involuntary micturition, leading to urinary incontinence. Neuroplasticity underlying these developmental and pathological changes in voiding function is discussed.
© 2014 American Physiological Society.
Figures
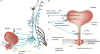















References
-
- Abdel-Gawad M, Dion SB, Elhilali MM. Evidence of a peripheral role of neurokinins in detrusor hyperreflexia: A further study of selective tachykinin antagonists in chronic spinal injured rats. J Urol. 2001;165:1739–1744. - PubMed
-
- Abdel-Karim AM, Barthlow HG, Bialecki RA, Elhilali MM. Effects of M274773, a neurokinin-2 receptor antagonist, on bladder function in chronically spinalized rats. J Urol. 2005;174:1488–1492. - PubMed
-
- Abe F. Inhibitory presynaptic effect of noradrenaline on the hypogastric ganglion of the guinea pig. J Pharmacol Exp Ther. 1984;231:395–403. - PubMed
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A, Standardisation Sub-committee of the International Continence S The standardisation of terminology of lower urinary tract function: Report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

