Autonomic control of the eye
- PMID: 25589275
- PMCID: PMC4919817
- DOI: 10.1002/cphy.c140014
Autonomic control of the eye
Abstract
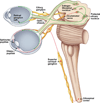
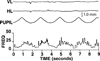
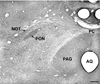
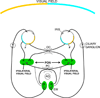
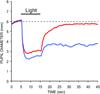
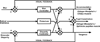
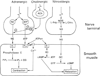
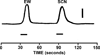
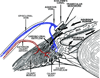
The autonomic nervous system influences numerous ocular functions. It does this by way of parasympathetic innervation from postganglionic fibers that originate from neurons in the ciliary and pterygopalatine ganglia, and by way of sympathetic innervation from postganglionic fibers that originate from neurons in the superior cervical ganglion. Ciliary ganglion neurons project to the ciliary body and the sphincter pupillae muscle of the iris to control ocular accommodation and pupil constriction, respectively. Superior cervical ganglion neurons project to the dilator pupillae muscle of the iris to control pupil dilation. Ocular blood flow is controlled both via direct autonomic influences on the vasculature of the optic nerve, choroid, ciliary body, and iris, as well as via indirect influences on retinal blood flow. In mammals, this vasculature is innervated by vasodilatory fibers from the pterygopalatine ganglion, and by vasoconstrictive fibers from the superior cervical ganglion. Intraocular pressure is regulated primarily through the balance of aqueous humor formation and outflow. Autonomic regulation of ciliary body blood vessels and the ciliary epithelium is an important determinant of aqueous humor formation; autonomic regulation of the trabecular meshwork and episcleral blood vessels is an important determinant of aqueous humor outflow. These tissues are all innervated by fibers from the pterygopalatine and superior cervical ganglia. In addition to these classical autonomic pathways, trigeminal sensory fibers exert local, intrinsic influences on many of these regions of the eye, as well as on some neurons within the ciliary and pterygopalatine ganglia.
© 2015 American Physiological Society.
Figures

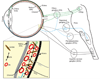
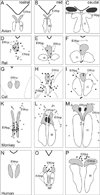
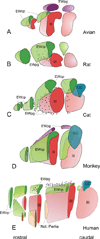
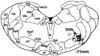








References
-
- Abe S, Karita K, Izumi H, Tamai M. Increased and Decreased Choroidal Blood Flow Elicited by Cervical Sympathetic Nerve Stimulation in the Cat. Jpn J Physiol. 1995;45:347–353. - PubMed
-
- Agassandian K, Fazan VPS, Adanina V, Talman WT. Direct Projections from the Cardiovascular Nucleus Tractus Solitarii to Pontine Preganglionic Parasympathetic Neurons: A Link to Cerebrovascular Regulation. J Comp Neurol. 2002;452:242–254. - PubMed
-
- Akao T, Mustari MJ, Fukushima J, Kurkin S, Fukushima K. Discharge Characteristics of Pursuit Neurons in Mst During Vergence Eye Movements. Journal of neurophysiology. 2005;93:2415–2434. - PubMed
-
- Akert K, Glicksman MA, Lang W, Grob P, Huber A. Edinger-Westphal Nucleus in the Monkey - Retrograde Tracer Study. Brain Research. 1980;184:491–498. - PubMed
-
- Alessandri M, Fusco BM, Maggi CA, Fanciullacci M. In Vivo Pupillary Constrictor Effects of Substance P in Man. Life sciences. 1991;48:2301–2308. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 EY001792/EY/NEI NIH HHS/United States
- R01 EY009380/EY/NEI NIH HHS/United States
- EY022290/EY/NEI NIH HHS/United States
- R01 EY007558/EY/NEI NIH HHS/United States
- EY007558/EY/NEI NIH HHS/United States
- P30 EY003039/EY/NEI NIH HHS/United States
- P30 EY03039/EY/NEI NIH HHS/United States
- GM103528/GM/NIGMS NIH HHS/United States
- EY09380/EY/NEI NIH HHS/United States
- P20 GM103528/GM/NIGMS NIH HHS/United States
- R01 EY014263/EY/NEI NIH HHS/United States
- R01 EY025555/EY/NEI NIH HHS/United States
- R01 EY022290/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

