The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination
- PMID: 25589426
- PMCID: PMC4295100
- DOI: 10.1038/srep07798
The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination
Abstract
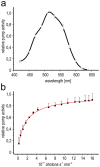
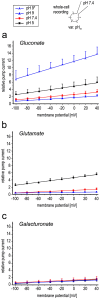
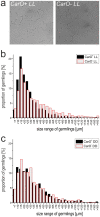
Rhodopsins are membrane-embedded photoreceptors found in all major taxonomic kingdoms using retinal as their chromophore. They play well-known functions in different biological systems, but their roles in fungi remain unknown. The filamentous fungus Fusarium fujikuroi contains two putative rhodopsins, CarO and OpsA. The gene carO is light-regulated, and the predicted polypeptide contains all conserved residues required for proton pumping. We aimed to elucidate the expression and cellular location of the fungal rhodopsin CarO, its presumed proton-pumping activity and the possible effect of such function on F. fujikuroi growth. In electrophysiology experiments we confirmed that CarO is a green-light driven proton pump. Visualization of fluorescent CarO-YFP expressed in F. fujikuroi under control of its native promoter revealed higher accumulation in spores (conidia) produced by light-exposed mycelia. Germination analyses of conidia from carO(-) mutant and carO(+) control strains showed a faster development of light-exposed carO(-) germlings. In conclusion, CarO is an active proton pump, abundant in light-formed conidia, whose activity slows down early hyphal development under light. Interestingly, CarO-related rhodopsins are typically found in plant-associated fungi, where green light dominates the phyllosphere. Our data provide the first reliable clue on a possible biological role of a fungal rhodopsin.
Figures





References
-
- Corrochano L. M. Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochem. Photobiol. Sci. 6, 725–736 (2007). - PubMed
-
- Bayram Ö., Braus G. H., Fischer R. & Rodríguez-Romero J. Spotlight on Aspergillus nidulans photosensory systems. Fungal Genet. Biol. 47, 900–908 (2010). - PubMed
-
- Avalos J. & Estrada A. F. Regulation by light in Fusarium. Fungal Genet. Biol. 47, 930–938 (2010). - PubMed
-
- Herrera-Estrella A. & Horwitz B. A. Looking through the eyes of fungi: molecular genetics of photoreception. Mol. Microbiol. 64, 5–15 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

