Lateral assembly of N-cadherin drives tissue integrity by stabilizing adherens junctions
- PMID: 25589573
- PMCID: PMC4345472
- DOI: 10.1098/rsif.2014.1055
Lateral assembly of N-cadherin drives tissue integrity by stabilizing adherens junctions
Abstract
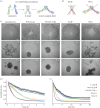
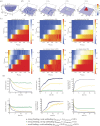
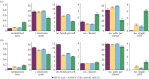
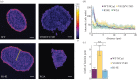
Cadherin interactions ensure the correct registry and anchorage of cells during tissue formation. Along the plasma membrane, cadherins form inter-junctional lattices via cis- and trans-dimerization. While structural studies have provided models for cadherin interactions, the molecular nature of cadherin binding in vivo remains unexplored. We undertook a multi-disciplinary approach combining live cell imaging of three-dimensional cell assemblies (spheroids) with a computational model to study the dynamics of N-cadherin interactions. Using a loss-of-function strategy, we demonstrate that each N-cadherin interface plays a distinct role in spheroid formation. We found that cis-dimerization is not a prerequisite for trans-interactions, but rather modulates trans-interfaces to ensure tissue stability. Using a model of N-cadherin junction dynamics, we show that the absence of cis-interactions results in low junction stability and loss of tissue integrity. By quantifying the binding and unbinding dynamics of the N-cadherin binding interfaces, we determined that mutating either interface results in a 10-fold increase in the dissociation constant. These findings provide new quantitative information on the steps driving cadherin intercellular adhesion and demonstrate the role of cis-interactions in junction stability.
Keywords: cell adhesion; data analysis; image analysis; long-term live cell imaging; mathematical modelling.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

