High preexisting serological antibody levels correlate with diversification of the influenza vaccine response
- PMID: 25589639
- PMCID: PMC4337521
- DOI: 10.1128/JVI.02871-14
High preexisting serological antibody levels correlate with diversification of the influenza vaccine response
Abstract
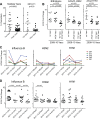
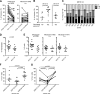
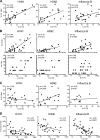
Reactivation of memory B cells allows for a rapid and robust immune response upon challenge with the same antigen. Variant influenza virus strains generated through antigenic shift or drift are encountered multiple times over the lifetime of an individual. One might predict, then, that upon vaccination with the trivalent influenza vaccine across multiple years, the antibody response would become more and more dominant toward strains consistently present in the vaccine at the expense of more divergent strains. However, when we analyzed the vaccine-induced plasmablast, memory, and serological responses to the trivalent influenza vaccine between 2006 and 2013, we found that the B cell response was most robust against more divergent strains. Overall, the antibody response was highest when one or more strains contained in the vaccine varied from year to year. This suggests that in the broader immunological context of viral antigen exposure, the B cell response to variant influenza virus strains is not dictated by the composition of the memory B cell precursor pool. The outcome is instead a diversified B cell response.
Importance: Vaccine strategies are being designed to boost broadly reactive B cells present in the memory repertoire to provide universal protection to the influenza virus. It is important to understand how past exposure to influenza virus strains affects the response to subsequent immunizations. The viral epitopes targeted by B cells responding to the vaccine may be a direct reflection of the B cell memory specificities abundant in the preexisting immune repertoire, or other factors may influence the vaccine response. Here, we demonstrate that high preexisting serological antibody levels to a given influenza virus strain correlate with low production of antibody-secreting cells and memory B cells recognizing that strain upon revaccination. In contrast, introduction of antigenically novel strains generates a robust B cell response. Thus, both the preexisting memory B cell repertoire and serological antibody levels must be taken into consideration in predicting the quality of the B cell response to new prime-boost vaccine strategies.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- F32AI93087/AI/NIAID NIH HHS/United States
- U19 AI090023/AI/NIAID NIH HHS/United States
- F32 AI009709/AI/NIAID NIH HHS/United States
- 1U19AI090023/AI/NIAID NIH HHS/United States
- 1P01AI09709/AI/NIAID NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- F32 AI093087/AI/NIAID NIH HHS/United States
- U19 AI109946/AI/NIAID NIH HHS/United States
- T32 AI007090/AI/NIAID NIH HHS/United States
- 1U19AI08724/AI/NIAID NIH HHS/United States
- P01 AI097092/AI/NIAID NIH HHS/United States
- 5U19AI057266/AI/NIAID NIH HHS/United States
- 5U54AI057158/AI/NIAID NIH HHS/United States
- U19 AI082724/AI/NIAID NIH HHS/United States
- T32AI007090/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

