Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease
- PMID: 25589660
- PMCID: PMC4403411
- DOI: 10.1128/JVI.03427-14
Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease
Abstract
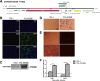
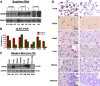
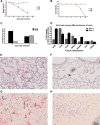
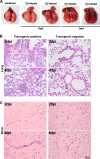
The emergence of Middle East respiratory syndrome-coronavirus (MERS-CoV) in the Middle East since 2012 has caused more than 900 human infections with ∼40% mortality to date. Animal models are needed for studying pathogenesis and for development of preventive and therapeutic agents against MERS-CoV infection. Nonhuman primates (rhesus macaques and marmosets) are expensive models of limited availability. Although a mouse lung infection model has been described using adenovirus vectors expressing human CD26/dipeptidyl peptidase 4 (DPP4), it is believed that a transgenic mouse model is needed for MERS-CoV research. We have developed this transgenic mouse model as indicated in this study. We show that transgenic mice globally expressing hCD26/DPP4 were fully permissive to MERS-CoV infection, resulting in relentless weight loss and death within days postinfection. High infectious virus titers were recovered primarily from the lungs and brains of mice at 2 and 4 days postinfection, respectively, whereas viral RNAs were also detected in the heart, spleen, and intestine, indicating a disseminating viral infection. Infected Tg(+) mice developed a progressive pneumonia, characterized by extensive inflammatory infiltration. In contrast, an inconsistent mild perivascular cuffing was the only pathological change associated with the infected brains. Moreover, infected Tg(+) mice were able to activate genes encoding for many antiviral and inflammatory mediators within the lungs and brains, coinciding with the high levels of viral replication. This new and unique transgenic mouse model will be useful for furthering knowledge of MERS pathogenesis and for the development of vaccine and treatments against MERS-CoV infection.
Importance: Small and economical animal models are required for the controlled and extensive studies needed for elucidating pathogenesis and development of vaccines and antivirals against MERS. Mice are the most desirable small-animal species for this purpose because of availability and the existence of a thorough knowledge base, particularly of genetics and immunology. The standard small animals, mice, hamsters, and ferrets, all lack the functional MERS-CoV receptor and are not susceptible to infection. So, initial studies were done with nonhuman primates, expensive models of limited availability. A mouse lung infection model was described where a mouse adenovirus was used to transfect lung cells for receptor expression. Nevertheless, all generally agree that a transgenic mouse model expressing the DPP4 receptor is needed for MERS-CoV research. We have developed this transgenic mouse model as indicated in this study. This new and unique transgenic mouse model will be useful for furthering MERS research.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276–278. doi: 10.1126/science.1087139. - DOI - PubMed
-
- Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H, Alhakeem R, Al-Tawfiq JA, Cotten M, Watson SJ, Kellam P, Zumla AI, Memish ZA. 2013. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 369:407–416. doi: 10.1056/NEJMoa1306742. - DOI - PMC - PubMed
-
- Guery B, Poissy J, el Mansouf L, Sejourne C, Ettahar N, Lemaire X, Vuotto F, Goffard A, Behillil S, Enouf V, Caro V, Mailles A, Che D, Manuguerra JC, Mathieu D, Fontanet A, van der Werf S. 2013. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet 381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

