Emergence and evolution of H10 subtype influenza viruses in poultry in China
- PMID: 25589662
- PMCID: PMC4403437
- DOI: 10.1128/JVI.03167-14
Emergence and evolution of H10 subtype influenza viruses in poultry in China
Abstract
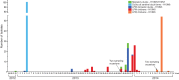
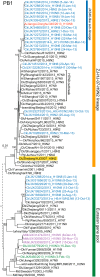
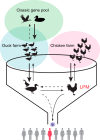
The cases of human infections with H10N8 viruses identified in late 2013 and early 2014 in Jiangxi, China, have raised concerns over the origin, prevalence, and development of these viruses in this region. Our long-term influenza surveillance of poultry and migratory birds in southern China in the past 12 years showed that H10 influenza viruses have been introduced from migratory to domestic ducks over several winter seasons at sentinel duck farms at Poyang Lake, where domestic ducks share their water body with overwintering migratory birds. H10 viruses were never detected in terrestrial poultry in our survey areas until August 2013, when they were identified at live-poultry markets in Jiangxi. Since then, we have isolated 124 H10N8 or H10N6 viruses from chickens at local markets, revealing an ongoing outbreak. Phylogenetic analysis of H10 and related viruses showed that the chicken H10N8 viruses were generated through multiple reassortments between H10 and N8 viruses from domestic ducks and the enzootic chicken H9N2 viruses. These chicken reassortant viruses were highly similar to the human isolate, indicating that market chickens were the source of human infection. Recently, the H10 viruses further reassorted, apparently with H5N6 viruses, and generated an H10N6 variant. The emergence and prevalence of H10 viruses in chickens and the occurrence of human infections provide direct evidence of the threat from the current influenza ecosystem in China.
Importance: After the outbreak of avian-origin H7N9 influenza viruses in China, fatal human infections with a novel H10N8 virus were reported. Utilizing data from 12 years of influenza surveillance in southern China, we showed that H10 viruses were regularly introduced by migratory ducks to domestic ducks on Poyang Lake, a major aggregative site of migratory birds in Asia. The H10 viruses were maintained and amplified in domestic ducks and then transmitted to chickens and reassorted with enzootic H9N2 viruses, leading to an outbreak and human infections at live-poultry markets. The emergence of the H10N8 virus, following a pathway similar to that of the recent H7N9 virus, highlights the role of domestic ducks and the current influenza ecosystem in China that facilitates influenza viruses moving from their reservoir hosts through the live-poultry system to cause severe consequences for public health.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Nguyen-Van-Tam JS, Nair P, Acheson P, Baker A, Barker M, Bracebridge S, Croft J, Ellis J, Gelletlie R, Gent N, Ibbotson S, Joseph C, Mahgoub H, Monk P, Reghitt TW, Sundkvist T, Sellwood C, Simpson J, Smith J, Watson JM, Zambon M, Lightfoot N, Incident Response Team . 2006. Outbreak of low pathogenicity H7N3 avian influenza in UK, including associated case of human conjunctivitis. Euro Surveill 11(18):pii=2952 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2952. - PubMed
-
- Fouchier RAM, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SAG, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJJ, Koch G, Bosman A, Koopmans M, Osterhaus ADME. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 101:1356–1361. doi:10.1073/pnas.0308352100. - DOI - PMC - PubMed
-
- Lam TT-Y, Wang J, Shen Y, Zhou B, Duan L, Cheung C-L, Ma C, Lycett SJ, Leung CY-H, Chen X, Li L, Hong W, Chai Y, Zhou L, Liang H, Ou Z, Liu Y, Farooqui A, Kelvin DJ, Poon LLM, Smith DK, Pybus OG, Leung GM, Shu Y, Webster RG, Webby RJ, Peiris JSM, Rambaut A, Zhu H, Guan Y. 2013. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 502:241–244. doi:10.1038/nature12515. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

