Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis
- PMID: 25589748
- PMCID: PMC4293409
- DOI: 10.1523/JNEUROSCI.2923-14.2015
Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis
Abstract
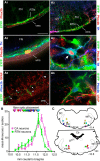
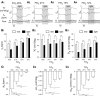
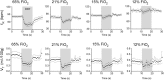
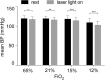
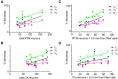
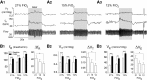
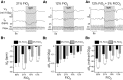
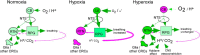
In conscious mammals, hypoxia or hypercapnia stimulates breathing while theoretically exerting opposite effects on central respiratory chemoreceptors (CRCs). We tested this theory by examining how hypoxia and hypercapnia change the activity of the retrotrapezoid nucleus (RTN), a putative CRC and chemoreflex integrator. Archaerhodopsin-(Arch)-transduced RTN neurons were reversibly silenced by light in anesthetized rats. We bilaterally transduced RTN and nearby C1 neurons with Arch (PRSx8-ArchT-EYFP-LVV) and measured the cardiorespiratory consequences of Arch activation (10 s) in conscious rats during normoxia, hypoxia, or hyperoxia. RTN photoinhibition reduced breathing equally during non-REM sleep and quiet wake. Compared with normoxia, the breathing frequency reduction (Δf(R)) was larger in hyperoxia (65% FiO2), smaller in 15% FiO2, and absent in 12% FiO2. Tidal volume changes (ΔV(T)) followed the same trend. The effect of hypoxia on Δf(R) was not arousal-dependent but was reversed by reacidifying the blood (acetazolamide; 3% FiCO2). Δf(R) was highly correlated with arterial pH up to arterial pH (pHa) 7.5 with no frequency inhibition occurring above pHa 7.53. Blood pressure was minimally reduced suggesting that C1 neurons were very modestly inhibited. In conclusion, RTN neurons regulate eupneic breathing about equally during both sleep and wake. RTN neurons are the first putative CRCs demonstrably silenced by hypocapnic hypoxia in conscious mammals. RTN neurons are silent above pHa 7.5 and increasingly active below this value. During hyperoxia, RTN activation maintains breathing despite the inactivity of the carotid bodies. Finally, during hypocapnic hypoxia, carotid body stimulation increases breathing frequency via pathways that bypass RTN.
Keywords: Archaerhodopsin; Phox2b; chemoreflex; medulla oblongata; optogenetics; ventrolateral medulla.
Copyright © 2015 the authors 0270-6474/15/350527-17$15.00/0.
Figures




References
-
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009b;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. - DOI - PMC - PubMed
-
- Abbott SB, DePuy SD, Nguyen T, Coates MB, Stornetta RL, Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci. 2013;33:3164–3177. doi: 10.1523/JNEUROSCI.1046-12.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
