A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes
- PMID: 25589779
- PMCID: PMC4564944
- DOI: 10.1136/jnnp-2014-309562
A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes
Abstract
Background: Patients presenting with parkinsonian syndromes share many clinical features, which can make diagnosis difficult. This is important as atypical parkinsonian syndromes (APSs) such as progressive supranuclear palsy (PSP), multiple system atrophy (MSA) and corticobasal syndrome (CBS) carry a poor prognosis, compared with patients with Parkinson's disease (PD). In addition, there is overlap between APS and dementia diseases, such as Alzheimer's disease (AD) and frontotemporal dementia (FTD).
Objective: To use a panel of cerebrospinal fluid (CSF) biomarkers to differentiate patients with APS from PD and dementia.
Methods: A prospective cohort of 160 patients and 30 control participants were recruited from a single specialist centre. Patients were clinically diagnosed according to current consensus criteria. CSF samples were obtained from patients with clinical diagnoses of PD (n=31), PSP (n=33), CBS (n=14), MSA (n=31), AD (n=26) and FTD (n=16). Healthy, elderly participants (n=30) were included as controls. Total τ (t-τ), phosphorylated τ (p-τ), β-amyloid 1-42 (Aβ42), neurofilament light chain (NFL), α-synuclein (α-syn), amyloid precursor protein soluble metabolites α and β (soluble amyloid precursor protein (sAPP)α, sAPPβ) and two neuroinflammatory markers (monocyte chemoattractant protein-1 and YKL-40) were measured in CSF. A reverse stepwise regression analysis and the false discovery rate procedure were used.
Results: CSF NFL (p<0.001), sAPPα (p<0.001) and a-syn (p=0.003) independently predicted diagnosis of PD versus APS. Together, these nine biomarkers could differentiate patients with PD from APS with an area under the curve of 0.95 and subtypes of APS from one another. There was good discriminatory power between parkinsonian groups, dementia disorders and healthy controls.
Conclusions: A panel of nine CSF biomarkers was able to differentiate APS from patients with PD and dementia. This may have important clinical utility in improving diagnostic accuracy, allowing better prognostication and earlier access to potential disease-modifying therapies.
Keywords: CORTICOBASAL DEGENERATION; CSF; PARKINSON'S DISEASE; POST MORTEM; SUPRANUCLEAR PALSY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


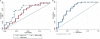

References
-
- Fahn S. Scientic Approaches Clin Neurol. 1977. Secondary Parkinsonism; pp. 1159–89.
-
- Ling H, O’Sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133:2045–57. doi:10.1093/brain/awq123. - PubMed
-
- Seltman RE, Matthews BR. Frontotemporal lobar degeneration: epidemiology, pathology, diagnosis and management. CNS Drugs. 2012;26:841–70. doi:10.2165/11640070-000000000-00000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
