Ocular drug delivery systems: An overview
- PMID: 25590022
- PMCID: PMC4289909
- DOI: 10.5497/wjp.v2.i2.47
Ocular drug delivery systems: An overview
Abstract
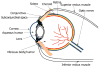
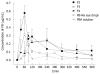
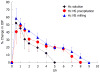
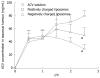
The major challenge faced by today's pharmacologist and formulation scientist is ocular drug delivery. Topical eye drop is the most convenient and patient compliant route of drug administration, especially for the treatment of anterior segment diseases. Delivery of drugs to the targeted ocular tissues is restricted by various precorneal, dynamic and static ocular barriers. Also, therapeutic drug levels are not maintained for longer duration in target tissues. In the past two decades, ocular drug delivery research acceleratedly advanced towards developing a novel, safe and patient compliant formulation and drug delivery devices/techniques, which may surpass these barriers and maintain drug levels in tissues. Anterior segment drug delivery advances are witnessed by modulation of conventional topical solutions with permeation and viscosity enhancers. Also, it includes development of conventional topical formulations such as suspensions, emulsions and ointments. Various nanoformulations have also been introduced for anterior segment ocular drug delivery. On the other hand, for posterior ocular delivery, research has been immensely focused towards development of drug releasing devices and nanoformulations for treating chronic vitreoretinal diseases. These novel devices and/or formulations may help to surpass ocular barriers and associated side effects with conventional topical drops. Also, these novel devices and/or formulations are easy to formulate, no/negligibly irritating, possess high precorneal residence time, sustain the drug release, and enhance ocular bioavailability of therapeutics. An update of current research advancement in ocular drug delivery necessitates and helps drug delivery scientists to modulate their think process and develop novel and safe drug delivery strategies. Current review intends to summarize the existing conventional formulations for ocular delivery and their advancements followed by current nanotechnology based formulation developments. Also, recent developments with other ocular drug delivery strategies employing in situ gels, implants, contact lens and microneedles have been discussed.
Keywords: Anatomy and physiology; Contact lens; Cornea; Drug delivery; Emulsions; Eye; Formulations; Implants; Liposomes; Nanomicelles; Ointments; Retina; Suspensions.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
