New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators
- PMID: 25590233
- PMCID: PMC4615062
- DOI: 10.1080/15384101.2014.995496
New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators
Abstract
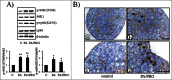
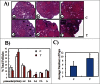
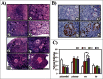
It had been known for decades that primordial follicles in mammalian ovaries are assembled with definite numbers and represent the ovarian reserve throughout the reproductive life. Intra-oocyte PI3K/mTOR pathways have been indicated to play a central role on the activation of primordial follicles. Genetic modified mouse models with chronic activation of PI3K/mTOR signals in primordial oocytes showed premature activation of all primordial follicles and eventually their exhaustion. On the other hand, this may suggest that, unlike chronic activation of PI3K/mTOR, its acute activation in infertility would activate primordial follicles, permitting fertility during the treatment. Previously, PI3K stimulators were reported as a temporary measure to accelerate primordial follicle activation and follicular development in both mouse and human, and were applied in the treatment of infertility in premature ovarian failure (POF) patients. To address whether mTOR stimulators could play similar role in the process, we transiently treated neonatal and aged mouse ovaries with mTOR stimulators-phosphatidic acid (PA) and propranolol. Our results demonstrated the stimulators increased activation of primordial follicles and the production of progeny. Human ovarian cortex cubes were also treated with mTOR or/and PI3K stimulators in vitro. When they were used separately, both of them showed similar promotive effects on primordial follicles. Surprisingly, after joint-treatment with the 2 kinds of stimulators together, synergistic effects on follicular development were observed. Based on increased efficiency of follicular activation in humans, here we propose in vitro transient treatment with mTOR and PI3K stimulators as an optimized protocol for the application in different clinical conditions with limited follicle reserve.
Keywords: PA, phosphatidic acid; POF, premature ovarian failure; PRO, propranolol.; PTEN, Phosphatase and Tensin Homolog deleted on chromosome 10; TSC1, tuberous sclerosis complex 1 or hamartin; TSC2, tuberous sclerosis complex 2 or tuberin; fertility preservation; follicular activation; mTOR, mammalian target of rapamycin; ovary; premature ovarian failure; primordial follicle.
Figures


References
-
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction 2009; 137:1-11; PMID:18827065; http://dx.doi.org/10.1530/REP-08-0118 - DOI - PubMed
-
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21:200-14; PMID:10782364 - PubMed
-
- Macklon NS, Fauser BC. Aspects of ovarian follicle development throughout life. Horm Res 1999; 52:161-70; PMID:10725781; http://dx.doi.org/10.1159/000023456 - DOI - PubMed
-
- Okeke T, Anyaehie U, Ezenyeaku C. Premature menopause. Ann Med Health Sci Res 2013; 3:90-5; PMID:23634337; http://dx.doi.org/10.4103/2141-9248.109458 - DOI - PMC - PubMed
-
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2005; 11:391-410; PMID:15919682; http://dx.doi.org/10.1093/humupd/dmi012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
