Bacterial flagella: twist and stick, or dodge across the kingdoms
- PMID: 25590430
- PMCID: PMC4295861
- DOI: 10.1371/journal.ppat.1004483
Bacterial flagella: twist and stick, or dodge across the kingdoms
Abstract
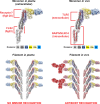
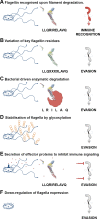
The flagellum organelle is an intricate multiprotein assembly best known for its rotational propulsion of bacteria. However, recent studies have expanded our knowledge of other functions in pathogenic contexts, particularly adherence and immune modulation, e.g., for Salmonella enterica, Campylobacter jejuni, Pseudomonas aeruginosa, and Escherichia coli. Flagella-mediated adherence is important in host colonisation for several plant and animal pathogens, but the specific interactions that promote flagella binding to such diverse host tissues has remained elusive. Recent work has shown that the organelles act like probes that find favourable surface topologies to initiate binding. An emerging theme is that more general properties, such as ionic charge of repetitive binding epitopes and rotational force, allow interactions with plasma membrane components. At the same time, flagellin monomers are important inducers of plant and animal innate immunity: variation in their recognition impacts the course and outcome of infections in hosts from both kingdoms. Bacteria have evolved different strategies to evade or even promote this specific recognition, with some important differences shown for phytopathogens. These studies have provided a wider appreciation of the functions of bacterial flagella in the context of both plant and animal reservoirs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

