Meiotic crossover patterns: obligatory crossover, interference and homeostasis in a single process
- PMID: 25590558
- PMCID: PMC4353236
- DOI: 10.4161/15384101.2014.991185
Meiotic crossover patterns: obligatory crossover, interference and homeostasis in a single process
Abstract
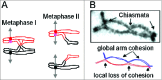
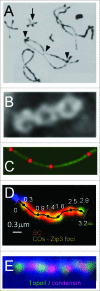
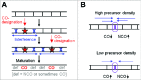
During meiosis, crossover recombination is tightly regulated. A spatial patterning phenomenon known as interference ensures that crossovers are well-spaced along the chromosomes. Additionally, every pair of homologs acquires at least one crossover. A third feature, crossover homeostasis, buffers the system such that the number of crossovers remains steady despite decreases or increases in the number of earlier recombinational interactions. Here we summarize recent work from our laboratory supporting the idea that all 3 of these aspects are intrinsic consequences of a single basic process and suggesting that the underlying logic of this process corresponds to that embodied in a particular (beam-film) model.
Keywords: BF, beam-film; CO, crossover; DDF, designation driving force; DSBs, double-strand breaks; NCO, noncrossover; SC, synaptonemal complex; STUbL, SUMO-targeted ubiquitin ligase; beam-film model; crossover; crossover homeostasis; crossover interference; meiosis; obligatory crossover; recombination.
Figures

References
-
- Kleckner N, Zhang L, Weiner B, Zickler D. Meiotic chromosome dynamics. In: Rippe, ed. Genome Organization and Function in the Mammalian Cell Nucleus. New York: John Wiley and Sons, 2011:487-534.
-
- Berchowitz LE, Copenhaver GP. Genetic interference: don't stand so close to me. Curr Genomics 2010; 11(2):91-102; PMID:20885817; http://dx.doi.org/10.2174/138920210790886835 - DOI - PMC - PubMed
-
- Jones GH, Franklin FC. Meiotic crossing-over: obligation and interference. Cell 2006; 126(2):246-8; PMID:16873056; http://dx.doi.org/10.1016/j.cell.2006.07.010 - DOI - PubMed
-
- Jones GH. The control of chiasma distribution. Symp Soc Exp Biol 1984; 38:293-320; PMID:6545727 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
