Discovery of CLC transport proteins: cloning, structure, function and pathophysiology
- PMID: 25590607
- PMCID: PMC4594286
- DOI: 10.1113/JP270043
Discovery of CLC transport proteins: cloning, structure, function and pathophysiology
Abstract
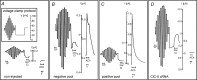
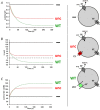
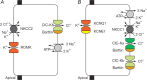
After providing a personal description of the convoluted path leading 25 years ago to the molecular identification of the Torpedo Cl(-) channel ClC-0 and the discovery of the CLC gene family, I succinctly describe the general structural and functional features of these ion transporters before giving a short overview of mammalian CLCs. These can be categorized into plasma membrane Cl(-) channels and vesicular Cl(-) /H(+) -exchangers. They are involved in the regulation of membrane excitability, transepithelial transport, extracellular ion homeostasis, endocytosis and lysosomal function. Diseases caused by CLC dysfunction include myotonia, neurodegeneration, deafness, blindness, leukodystrophy, male infertility, renal salt loss, kidney stones and osteopetrosis, revealing a surprisingly broad spectrum of biological roles for chloride transport that was unsuspected when I set out to clone the first voltage-gated chloride channel.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures
References
-
- Accardi A. Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. - PubMed
-
- Adachi S, Uchida S, Ito H, Hata M, Hiroe M, Marumo F. Sasaki S. Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle’s loop and collecting ducts of rat kidney. J Biol Chem. 1994;269:17677–17683. - PubMed
-
- Akizuki N, Uchida S, Sasaki S. Marumo F. Impaired solute accumulation in inner medulla of Clcnk1−/− mice kidney. Am J Physiol Renal Physiol. 2001;280:F79–F87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

