Reprogramming of Yersinia from virulent to persistent mode revealed by complex in vivo RNA-seq analysis
- PMID: 25590628
- PMCID: PMC4295882
- DOI: 10.1371/journal.ppat.1004600
Reprogramming of Yersinia from virulent to persistent mode revealed by complex in vivo RNA-seq analysis
Erratum in
-
Correction: Reprogramming of Yersinia from virulent to persistent mode revealed by complex in vivo RNA-seq analysis.PLoS Pathog. 2015 Mar 25;11(3):e1004769. doi: 10.1371/journal.ppat.1004769. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25807375 Free PMC article. No abstract available.
Abstract
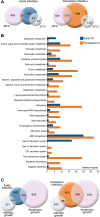
We recently found that Yersinia pseudotuberculosis can be used as a model of persistent bacterial infections. We performed in vivo RNA-seq of bacteria in small cecal tissue biopsies at early and persistent stages of infection to determine strategies associated with persistence. Comprehensive analysis of mixed RNA populations from infected tissues revealed that Y. pseudotuberculosis undergoes transcriptional reprogramming with drastic down-regulation of T3SS virulence genes during persistence when the pathogen resides within the cecum. At the persistent stage, the expression pattern in many respects resembles the pattern seen in vitro at 26oC, with for example, up-regulation of flagellar genes and invA. These findings are expected to have impact on future rationales to identify suitable bacterial targets for new antibiotics. Other genes that are up-regulated during persistence are genes involved in anaerobiosis, chemotaxis, and protection against oxidative and acidic stress, which indicates the influence of different environmental cues. We found that the Crp/CsrA/RovA regulatory cascades influence the pattern of bacterial gene expression during persistence. Furthermore, arcA, fnr, frdA, and wrbA play critical roles in persistence. Our findings suggest a model for the life cycle of this enteropathogen with reprogramming from a virulent to an adapted phenotype capable of persisting and spreading by fecal shedding.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

