PKR downregulation prevents neurodegeneration and β-amyloid production in a thiamine-deficient model
- PMID: 25590804
- PMCID: PMC4669750
- DOI: 10.1038/cddis.2014.552
PKR downregulation prevents neurodegeneration and β-amyloid production in a thiamine-deficient model
Abstract
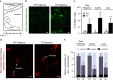
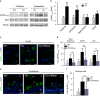
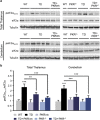
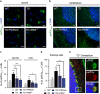
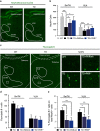
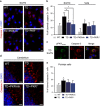
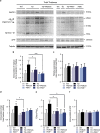
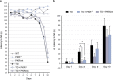
Brain thiamine homeostasis has an important role in energy metabolism and displays reduced activity in Alzheimer's disease (AD). Thiamine deficiency (TD) induces regionally specific neuronal death in the animal and human brains associated with a mild chronic impairment of oxidative metabolism. These features make the TD model amenable to investigate the cellular mechanisms of neurodegeneration. Once activated by various cellular stresses, including oxidative stress, PKR acts as a pro-apoptotic kinase and negatively controls the protein translation leading to an increase of BACE1 translation. In this study, we used a mouse TD model to assess the involvement of PKR in neuronal death and the molecular mechanisms of AD. Our results showed that the TD model activates the PKR-eIF2α pathway, increases the BACE1 expression levels of Aβ in specific thalamus nuclei and induces motor deficits and neurodegeneration. These effects are reversed by PKR downregulation (using a specific inhibitor or in PKR knockout mice).
Conflict of interest statement
Professor Jacques Hugon is a consultant for Roche, Sanofi, Novartis, Xigen, Eisai and Lundbeck. Dr. Claire Paquet is a consultant for Lilly and Novartis. Dr. Julien Dumurgier is a consultant for Novartis. Dr. Patrick Bernardelli, Dr. Véronique Taupin, Dr. Thomas Rooney and Dr. Laurent Pradier are fully employed by Sanofi. The remaining authors declare no conflict of interest.
Figures
References
-
- Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience 1999; 91: 429–438. - PubMed
-
- Calingasan NY, Huang PL, Chun HS, Fabian A, Gibson GE. Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol Exp Neurol 2000; 59: 207–217. - PubMed
-
- Ke ZJ, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem Int 2004; 45: 361–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

