Dose-dependent effects of selenite (Se(4+)) on arsenite (As(3+))-induced apoptosis and differentiation in acute promyelocytic leukemia cells
- PMID: 25590806
- PMCID: PMC4669761
- DOI: 10.1038/cddis.2014.563
Dose-dependent effects of selenite (Se(4+)) on arsenite (As(3+))-induced apoptosis and differentiation in acute promyelocytic leukemia cells
Abstract
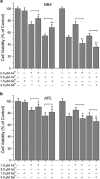
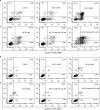
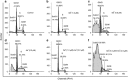
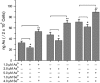
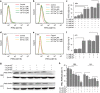
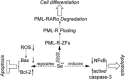
To enhance the therapeutic effects and decrease the adverse effects of arsenic on the treatment of acute promyelocytic leukemia, we investigated the co-effects of selenite (Se(4+)) and arsenite (As(3+)) on the apoptosis and differentiation of NB4 cells and primary APL cells. A 1.0-μM concentration of Se(4+) prevented the cells from undergoing As(3+)-induced apoptosis by inhibiting As(3+) uptake, eliminating As(3+)-generated reactive oxygen species, and repressing the mitochondria-mediated intrinsic apoptosis pathway. However, 4.0 μM Se(4+) exerted synergistic effects with As(3+) on cell apoptosis by promoting As(3+) uptake, downregulating nuclear factor-кB, and activating caspase-3. In addition to apoptosis, 1.0 and 3.2 μM Se(4+) showed contrasting effects on As(3+)-induced differentiation in NB4 cells and primary APL cells. The 3.2 μM Se(4+) enhanced As(3+)-induced differentiation by promoting the degradation of promyelocytic leukemia protein-retinoic acid receptor-α (PML-RARα) oncoprotein, but 1.0 μM Se(4+) did not have this effect. Based on mechanistic studies, Se(4+), which is similar to As(3+), might bind directly to Zn(2+)-binding sites of the PML RING domain, thus controlling the fate of PML-RARα oncoprotein.
Figures



References
-
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 2008; 111: 2505–2515. - PubMed
-
- De Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature 1990; 347: 558–561. - PubMed
-
- Zhu J, Lallemand-Breitenbach V, de Thé H. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARα catabolism, role of oncogene degradation in disease remission. Oncogene 2001; 20: 7257–7265. - PubMed
-
- Lengfelder E, Hofmann WK, Nowak D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 2012; 26: 433–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

