Macrophage peroxisome proliferator-activated receptor γ deficiency delays skin wound healing through impairing apoptotic cell clearance in mice
- PMID: 25590807
- PMCID: PMC4669743
- DOI: 10.1038/cddis.2014.544
Macrophage peroxisome proliferator-activated receptor γ deficiency delays skin wound healing through impairing apoptotic cell clearance in mice
Abstract
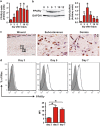
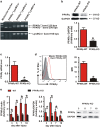
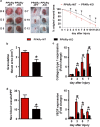
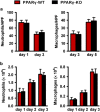
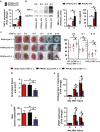
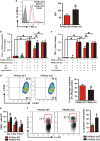
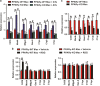
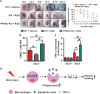
Skin wound macrophages are key regulators of skin repair and their dysfunction causes chronic, non-healing skin wounds. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates pleiotropic functions of macrophages, but its contribution in skin wound healing is poorly defined. We observed that macrophage PPARγ expression was upregulated during skin wound healing. Furthermore, macrophage PPARγ deficiency (PPARγ-knock out (KO)) mice exhibited impaired skin wound healing with reduced collagen deposition, angiogenesis and granulation formation. The tumor necrosis factor alpha (TNF-α) expression in wounds of PPARγ-KO mice was significantly increased and local restoration of TNF-α reversed the healing deficit in PPARγ-KO mice. Wound macrophages produced higher levels of TNF-α in PPARγ-KO mice compared with control. In vitro, the higher production of TNF-α by PPARγ-KO macrophages was associated with impaired apoptotic cell clearance. Correspondingly, increased apoptotic cell accumulation was found in skin wound of PPARγ-KO mice. Mechanically, peritoneal and skin wound macrophages expressed lower levels of various phagocytosis-related molecules. In addition, PPARγ agonist accelerated wound healing and reduced local TNF-α expression and wound apoptotic cells accumulation in wild type but not PPARγ-KO mice. Therefore, PPARγ has a pivotal role in controlling wound macrophage clearance of apoptotic cells to ensure efficient skin wound healing, suggesting a potential new therapeutic target for skin wound healing.
Figures
References
-
- Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology 2011; 216: 753–762. - PubMed
-
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007; 25: 9–18. - PubMed
-
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999; 341: 738–746. - PubMed
-
- Natarajan S, Williamson D, Stiltz AJ, Harding K. Advances in wound care and healing technology. Am J Clin Dermatol 2000; 1: 269–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

