In vitro activity of the hydroethanolic extract and biflavonoids isolated from Selaginella sellowii on Leishmania (Leishmania) amazonensis
- PMID: 25591109
- PMCID: PMC4325620
- DOI: 10.1590/0074-0276140312
In vitro activity of the hydroethanolic extract and biflavonoids isolated from Selaginella sellowii on Leishmania (Leishmania) amazonensis
Abstract
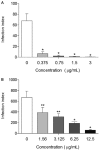
This study is the first phytochemical investigation of Selaginella sellowii and demonstrates the antileishmanial activity of the hydroethanolic extract from this plant (SSHE), as well as of the biflavonoids amentoflavone and robustaflavone, isolated from this species. The effects of these substances were evaluated on intracellular amastigotes of Leishmania (Leishmania) amazonensis, an aetiological agent of American cutaneous leishmaniasis. SSHE was highly active against intracellular amastigotes [the half maximum inhibitory concentration (IC50) = 20.2 µg/mL]. Fractionation of the extract led to the isolation of the two bioflavonoids with the highest activity: amentoflavone, which was about 200 times more active (IC50 = 0.1 μg/mL) and less cytotoxic than SSHE (IC50 = 2.2 and 3 μg/mL, respectively on NIH/3T3 and J774.A1 cells), with a high selectivity index (SI) (22 and 30), robustaflavone, which was also active against L. amazonensis (IC50 = 2.8 µg/mL), but more cytotoxic, with IC50 = 25.5 µg/mL (SI = 9.1) on NIH/3T3 cells and IC50 = 3.1 µg/mL (SI = 1.1) on J774.A1 cells. The production of nitric oxide (NO) was lower in cells treated with amentoflavone (suggesting that NO does not contribute to the leishmanicidal mechanism in this case), while NO release was higher after treatment with robustaflavone. S. sellowii may be a potential source of biflavonoids that could provide promising compounds for the treatment of cutaneous leishmaniasis.
Figures



References
-
- Agrawal PK, Bansal MC. Other Flavonoids. In: Agrawal PK, editor. Carbon-13 NMR of flavonoids. Studies in organic chemistry-39. Elsevier; Amsterdam: 1989. pp. 236–282.
-
- Assis ELM, Labiak PH. Lycophyta da borda oeste do Pantanal, Mato Grosso do Sul, Brasil. Acta Bot Bras. 2009;23:703–712.
-
- Banerjee T, Van der Vliet A, Ziboh VA. Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins Leukot Essent Fatty Acids. 2002;66:485–492. - PubMed
-
- Brahmachari G. Brahmachari G. Bioactive natural products: opportunities and challenges in medicinal chemistry. 1st. World Scientific Publishing Co. Pte. Ltd.; Singapore: 2011. Natural products in drug discovery: impacts and opportunities - an assessment; pp. 1–199.
-
- Camacho MR, Mata R, Castaneda P, Kirby GC, Warhurst DC, Croft SL, Phillipson JD. Bioactive compounds from Celaenodendron mexicanum. Planta Med. 2000;66:463–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

