Linking protein motion to enzyme catalysis
- PMID: 25591120
- PMCID: PMC4341894
- DOI: 10.3390/molecules20011192
Linking protein motion to enzyme catalysis
Abstract
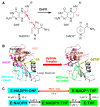
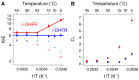
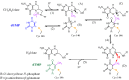
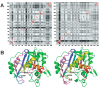
Enzyme motions on a broad range of time scales can play an important role in various intra- and intermolecular events, including substrate binding, catalysis of the chemical conversion, and product release. The relationship between protein motions and catalytic activity is of contemporary interest in enzymology. To understand the factors influencing the rates of enzyme-catalyzed reactions, the dynamics of the protein-solvent-ligand complex must be considered. The current review presents two case studies of enzymes-dihydrofolate reductase (DHFR) and thymidylate synthase (TSase)-and discusses the role of protein motions in their catalyzed reactions. Specifically, we will discuss the utility of kinetic isotope effects (KIEs) and their temperature dependence as tools in probing such phenomena.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

