Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study
- PMID: 25591505
- PMCID: PMC4427052
- DOI: 10.1016/S0140-6736(14)61228-9
Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study
Abstract
Background: Direct-acting antiviral drugs have a high cure rate and favourable tolerability for patients with hepatitis C virus (HCV). Shorter courses could improve affordability and adherence. Sofosbuvir and ledipasvir with ribavirin have high efficacy when taken for 8 weeks but not for 6 weeks. We assessed whether the addition of a third direct-acting antiviral drug to sofosbuvir and ledipasvir would allow a shorter treatment duration.
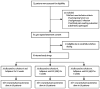
Methods: In this single-centre, open-label, phase 2A trial, we sequentially enrolled treatment-naive patients with HCV genotype 1 infection into three treatment groups: 12 weeks of sofosbuvir and ledipasvir; 6 weeks of sofosbuvir, ledipasvir, and GS-9669; or 6 weeks of sofosbuvir, ledipasvir, and GS-9451. Patients and investigators were not masked to treatment assignment. The primary endpoint was the propotion of patients with sustained viral response at 12 weeks after treatment completion (SVR12), assessed by serum HCV RNA concentrations lower than 43 IU/mL (the lower limit of quantification). We did an intention-to-treat analysis for the primary endpoint and adverse events. This study is registered with ClinicalTrials.gov, number NCT01805882.
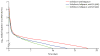
Findings: Between Jan 11, 2013, and Dec 17, 2013, we enrolled 60 patients, and sequentially assigned them into three groups of 20. We noted an SVR12 in all 20 patients (100%, 95% CI 83-100) allocated to sofosbuvir and ledipasvir for 12 weeks; in 19 (95%, 75-100) of the 20 patients allocated to sofosbuvir, ledipasvir, and GS-9669 for 6 weeks (one patient relapsed 2 weeks after completion of treatment); and in 19 (95%, 75-100%) of the 20 patients allocated to sofosbuvir, ledipasvir, and GS-9451 for 6 weeks (one patient was lost to follow-up after reaching sustained viral response at 4 weeks). Most adverse events were mild and no patients discontinued treatment. Two serious adverse events occurred (pain after a post-treatment liver biopsy and vertigo), both unrelated to study drugs.
Interpretation: In this small proof-of-concept study, two different three-drug regimens that were given for 6 weeks resulted in high cure rates for HCV infection with excellent tolerability. Addition of a third potent direct-acting antiviral drug can reduce the duration of treatment required to achieve sustained viral response in patients with chronic HCV genotype 1 infection without cirrhosis.
Funding: National Institute of Allergy and Infectious Diseases (NIAID), National Cancer Institute and Clinical Center Intramural Program, German Research Foundation, National Institutes of Health, Gilead Sciences.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
William T. Symonds, Phillip Pang, G. Mani Subramanian and John G. McHutchison are employees of Gilead Pharmaceuticals. Rohit Talwani has served as a speaker for Merck and performs research funded by Vertex pharmaceuticals. Jose Chavez is a Member of the Regional Advisory Boards for Abbott, Bristol-Myers Squibb and Gilead. Gebeyehu Teferi serves on the Gilead and Merck Advisory Boards and as a speaker for Gilead.
Figures
Comment in
-
Shorter treatments for hepatitis C: another step forward?Lancet. 2015 Mar 21;385(9973):1054-5. doi: 10.1016/S0140-6736(14)61600-7. Epub 2015 Jan 13. Lancet. 2015. PMID: 25591504 No abstract available.
References
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. - PubMed
-
- Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Seminars in liver disease. 2000;20(1):17–35. - PubMed
-
- Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, Demicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;13(5):401–8. - PubMed
-
- Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

