A lacZ reporter gene expression atlas for 313 adult KOMP mutant mouse lines
- PMID: 25591789
- PMCID: PMC4381530
- DOI: 10.1101/gr.184184.114
A lacZ reporter gene expression atlas for 313 adult KOMP mutant mouse lines
Abstract
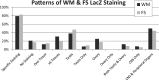
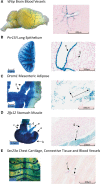
Expression of the bacterial beta-galactosidase reporter gene (lacZ) in the vector used for the Knockout Mouse Project (KOMP) is driven by the endogenous promoter of the target gene. In tissues from KOMP mice, histochemical staining for LacZ enzyme activity can be used to determine gene expression patterns. With this technique, we have produced a comprehensive resource of gene expression using both whole mount (WM) and frozen section (FS) LacZ staining in 313 unique KOMP mutant mouse lines. Of these, ∼ 80% of mutants showed specific staining in one or more tissues, while ∼ 20% showed no specific staining, ∼ 13% had staining in only one tissue, and ∼ 25% had staining in >6 tissues. The highest frequency of specific staining occurred in the brain (∼ 50%), male gonads (42%), and kidney (39%). The WM method was useful for rapidly identifying whole organ and some substructure staining, while the FS method often revealed substructure and cellular staining specificity. Both staining methods had >90% repeatability in biological replicates. Nonspecific LacZ staining occurs in some tissues due to the presence of bacteria or endogenous enzyme activity. However, this can be effectively distinguished from reporter gene activity by the combination of the WM and FS methods. After careful annotation, LacZ staining patterns in a high percentage of mutants revealed a unique structure-function not previously reported for many of these genes. The validation of methods for LacZ staining, annotation, and expression analysis reported here provides unique insights into the function of genes for which little is currently known.
© 2015 West et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Adams AC, Gale NW. 2006. High resolution gene expression analysis in mice using genetically inserted reporter genes. In Mammalian and avian transgenesis: new approaches (principles and practice) (ed. Pease S, Lois C), pp. 131–161 Springer-Verlag, Berlin.
-
- Casadaban MJ, Chou J, Cohen SN. 1980. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol 143: 971–980. - PMC - PubMed
-
- Gates H, Mallon AM, Brown SD; EUMODIC Consortium. 2011. High-throughput mouse phenotyping. Methods 53: 394–404. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
